KCl
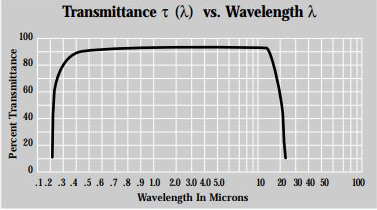
Potassium Chloride (KCl) is hygrosopic. The crystal often applied for infared transmission windows and FTIR spectrophotometers. The KCl windows have low refractive index and high threshold damage. So it’s very useful for sputter barrier windows in CO2 lasers. KCl single crystal uniform texture and transparent, has a high infrared transmittance, the transmittance greater than 90% when thickness less 10mm and in the range of 4000-500cm-1, no impurity absorption. KCI single crystal as a laser window material, the optical performance is excellent.
Parameter
| Orientation | <100>, <110>, <111> |
| Orientation Tolerance | < 0.5° |
| Parallelism | 5〞 |
| Perpendicularity | 3ˊ |
| Surface Quality | 10-5 (Scratch/Dig) |
| Wavefront Distortion | <λ/4@632 nm |
| Surface Flatness | <λ/8 @632 nm |
| Clear Aperture | >90% |
| Chamfer | <0.1×45° |
| Thickness/Diameter Tolerance | ±0.05 mm |
| Crystal Structure | Cubic |
| Symmetry Class | m3m |
| Lattice Constants | 6.291 Å |
| Density | 1.989 g/cm3 |
| Melting Point | 776°C |
| Cleavability | (100), perfect |
| Thermal Conductivity /(W·m-1·K-1)@46°C | 6.53 |
| Specific Heat (J·kg-1·K-1) | 690 |
| Thermal Expansion(10-6·K-1@60°C) | 34.1 … 38.3 |
| Hardness (Knoop) | 7.2@<110>, 9.3@<100> |
| Vickers Microhardness (GPa) | 0.15 |
| Young’s Modulus (GPa) | 16.8@<110>, 38.2@<100> |
| Shear Modulus (GPa) | 6.3@<110>, 10.8@<100> |
| Bulk Modulus (GPa) | 17.36 |
| Rupture Modulus (GPa) | 4.41×10-3 |
| Elastic Coefficient (GPa) | C11=40.2, C12=6.7, C44=6.29 |
| Transmission Range | 0.21 … 30µm |
| Refractive Index | 1.488@0.6µm, 1.454@10.6µm |
| Reflective Loss | 6.8%@10.0µm |
| Reststrahlen | 63.1µm |
| Poisson Ratio | 0.134 |
| λ(μm) | n | λ(μm) | n | λ(μm) | n |
| 0.2 | 1.717 | 5 | 1.4703 | 11 | 1.4527 |
| 0.5 | 1.4968 | 6 | 1.4683 | 12 | 1.4463 |
| 1 | 1.4796 | 7 | 1.4659 | 12.5 | 1.446 |
| 2 | 1.4751 | 8 | 1.4632 | 15 | 1.4325 |
| 3 | 1.4735 | 9 | 1.4601 | 20 | 1.3947 |
| 4 | 1.472 | 10 | 1.4566 | 30 | 1.2626 |
Feature
Application
Literature
Feature
- Wide-band good conductor
- Water-soluble, easy to deliquesce and cannot be chemically polished
- Can grow epitaxial films on a featureless substrate
Application
- Substrate for Epitaxial growth
- Be used in the production of infrared spectroscopy analyzer
- Ultraviolet and infrared optical components
- Prisms, lenses, filters and various laser windows, infrared devices, optical, laser crystal instruments
- IR spectroscopy
- Windows for CO2lasers
- Protection windows for cutting lenses
Literature
| [1] Xu C, Jiao C, Yao R,et al. Adsorption and regeneration of expanded graphite modified by CTAB-KBr/H3PO4 for marine oil pollution[J]. Environmental Pollution, 2017, 233:194-200. |
| [2] Hongen, Gu, and, et al. Electrolytic coloration and spectral properties of OH−-doped KBr polycrystals[J]. Physica B: Condensed Matter, 2009. |
| [3] Cui R Z , Yang L , Zhang T T , et al. Measurements and calculations of solid–liquid equilibria in the quaternary system NaBr–KBr–Na2SO4–K2SO4–H2O at 298K[J]. Calphad-computer Coupling of Phase Diagrams & Thermochemistry, 2016, 54:117-124. |
| [4] Halimi O , Boudine B , Sebais M , et al. Structural and optical characterisation of ZnO nanocrystals embedded in bulk KBr single crystal[J]. Materials Science & Engineering C, 2003, 23(6-8):1111-1114. |
| [5] Hiroyoshi, Nagae, and, et al. Mechanism enabling the observation of the formally optically-forbidden and states in resonance-Raman excitation profiles of spheroidene in KBr disc[J]. Chemical Physics, 2010, 373(1-2):145-152. |
| [6] Goswami S , Devaraju B , Weigelt M , et al. Analysis of GRACE range-rate residuals with focus on KBR instrument system noise[J]. Advances in Space Research, 2017. |
| [7] A. Marcinkeviiūt, V . Jukna, Uminas R , et al. Supercontinuum generation in the absence and in the presence of color centers in NaCl and KBr[J]. Results in Physics, 14(C):102396-102396. |
| [8] Beaudoin N , Hamilton A , Koehn D , et al. Reaction-induced porosity fingering: replacement dynamic and porosity evolution in the KBr-KCl system[J]. Geochimica Et Cosmochimica Acta, 2018:S0016703718302424. |
| [9] A J L , B M B , B J O L . Production and use of radioactive [82Br]KBr in high-temperature corrosion studies – ScienceDirect[J]. Corrosion Science, 2019, 148:24-30. |
| [10] Bensouici A , Plaza J L , Dieguez E , et al. Optical and structural characterization of KBr crystals doped cadmium bromide (CdBr 2)[J]. Journal of Luminescence, 2010, 130(4):688-691. |
| [11] Glna B , Rzca B , Shsa B , et al. Experimental study and theoretical simulation of fluid phase equilibrium in the subsystems of quinary system NaBr–KBr–MgBr2–SrBr2–H2O at 298K – ScienceDirect[J]. Journal of Molecular Liquids, 306. |
| [12] Rui-Zhi, Cui, Zhou-Chi, et al. Measurements and calculations of solid-liquid equilibria in the quaternary system KBr–CaBr2–MgBr2–H2O at (298 and 323) K[J]. Fluid Phase Equilibria, 2017. |
| [13] Rm A , Go B , Fm A . KBR (Kinetics in Batch Reactors): a MATLAB-based application with a friendly Graphical User Interface for chemical kinetic model simulation and parameter estimation[J]. Education for Chemical Engineers, 2019, 28:80-89. |
| [14] Zeid I , El-Kork N , Korek M . Electronic structure with the calculation of the rovibrational, and dipole moments of the electronic states of the NaBr and KBr molecules[J]. Chemical Physics, 2019, 517:36-47. |
| [15] Elimination of interference from water in KBr disk FT-IR spectra of solid biomaterials by chemometrics solved with kinetic modeling[J]. Talanta, 2017, 174:587. |
| [16] Matsunami N , Okayasu S , Sataka M , et al. Electronic sputtering of SiC and KBr by high energy ions[J]. Nuclear Instruments and Methods in Physics Research Section B Beam Interactions with Materials and Atoms, 2020, 478:80-84. |
| [17] P Ciochoń, Olszowska N , Koodziej J J . Electronic structure and STM imaging of the KBr-InSb interface[J]. Applied Surface Science, 2017, 409:200-207. |
| [18] Bouhdjer L , Addala S , Halimi O , et al. CuO nanocrystals embedded in KBr single crystal: Elaboration and Characterization[J]. Optik – International Journal for Light and Electron Optics, 2017, 145:99-105. |
| [19] Zhang H , Wang X , Li Y . Measuring radiative properties of silica aerogel composite from FTIR transmittance test using KBr as diluents[J]. Experimental Thermal & Fluidence, 2018:S0894177717303059. |
| [20] Fuentes-Azcatl R , Barbosa M C . Potassium bromide, KBr/ ε : New Force Field[J]. Physica A: Statistical Mechanics and its Applications, 2018, 491:480-489. |
| [21] Zhao G , Tong R . Silica gel enables Achmatowicz rearrangement with KBr/oxone under “anhydrous” condition for one-pot functionalization[J]. Tetrahedron, 2018. |
| [22] A Caló, Huttula M , Patanen M , et al. Valence photoelectron spectrum of KBr: Effects of electron correlation[J]. Journal of Electron Spectroscopy & Related Phenomena, 2008, 162(1):30-35. |
| [23] Yuan N , Sun H X , Zhang Z B , et al. Performance Improvement of Temperature Sensor Based on Rare-earth Ion Fluorescence by Using KBr-diluted Phosphor[J]. Materials Letters, 2016, 185(dec.15):573-575. |
| [24] Fukazawa Y, Tasaki K, Susuki Y.Oscillating behavior of proton scattering amplitude on an electron-stimulated-desorbed surface of KBr by keV electrons[J]. Nuclear Instruments and Methods in Physics Research Section B Beam Interactions with Materials and Atoms, 2020, 478:125-130. |
| [25] Rai R , Triloki, Singh B K , et al. Correlation between photoemissive and morphological properties of KBr thin film photocathodes[J]. Nuclear Instruments & Methods in Physics Research, 2017, 912(DEC.21):6-10. |
| [26] Bensouici A , Plaza J L , E Diéguez, et al. CdTe aggregates in KBr crystalline matrix[J]. Journal of Luminescence, 2009, 129(9):948-951. |
| [27] Lin, Hualin, Liu, et al. Mixed and ground KBr-impregnated calcined snail shell and kaolin as solid base catalysts for biodiesel production[J]. Renewable Energy, 2016. |
| [28] Reddeppa N, Sharma A K , Rao V,et al. Preparation and characterization of pure and KBr doped polymer blend (PVC/PEO) electrolyte thin films[J]. Microelectronic Engineering, 2013, 112(dec.):57-62. |
| [29] Gong W, Gaune-Escard M . A thermodynamic description of the KBr–EuBr2 system[J]. Journal of Alloys & Compounds, 2014, 584:503-506. |
| [30] Mrm A , Bm A , Skm A , et al. Dioxidomolybdenum(VI) and dioxidouranium(VI) complexes as functional mimic of haloperoxidases catalytic activity in presence of H 2 O 2 –KBr–HClO 4[J]. Inorganica Chimica Acta, 2019, 486:757-765. |



Leave a Reply