Ce:LiSAF
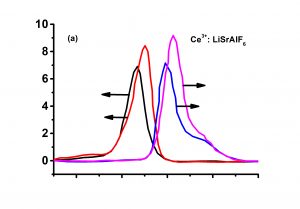
Lithium strontium hexafluoroaluminate (LiSrAlF6, LiSAF) are excellent host materials for tunable all-solid-state lasers in the UV region when doped with trivalent cerium(Ce:LiSAF).The gain spectra of Ce:LiSAF is in the range 280-320 nm and is characteristic of the Ce3+ 5d1–4f1 interconfigurational transition. Ce:LiSAF is attractive UV solid-state laser materials with the central emission wavelength at 290 nm and a practical tuning range from 288 to 315 nm. The slope efficiencies of Ce:LiSAF has been reported to reach as high as 29%. The broad gain-bandwidth of this fluoride crystals in the UV region has made it appealing for ultrashort-pulse generation and amplification. It can also be pumped by the fourth harmonic of a Nd:YAG laser. Ce:LiSAF is the preferred material of the colquiriite hosts, since it shows higher gains than Ce:LiCAF.
Parameter
| Orientation Tolerance | 5ˊ |
| Parallelism | <10〞 |
| Perpendicularity | 5ˊ |
| Chamfer | 0.1mm@45° |
| Surface Quality | 10/5 or better |
| Wavefront Distortion | λ/8 @632.8 nm |
| Surface Flatness | λ/10 @632.8 nm |
| Clear Aperture | >95% |
| Diameter Tolerance | +0/-0.05mm |
| Length Tolerance | ±0.1mm |
| Coatings | As per requirement |
| Dopant Concentration Tolerance | 0.10% |
| Crystal Structure | Trigonal |
| Space Group | P31C |
| Lattice Constants | a=5.08, c=10.15Å@1mol%CeF3 |
| Density (g/cm3) | 3.45 |
| Melting Point | 766°C |
| Thermal Conductivity(W·m-1·K-1) | 3.1 |
| Thermal Expansion(10-6K-1) | 21.6(∥a),-6.7(∥c) |
| Absorption Cross-section(10-18cm2)@266nm | 7.3(π), 6.6(σ) |
| Absorption Coefficient@266nm | 7cm-1 |
| Refractive Index | n=1.42 |
| Peak Lasing Wavelength(nm) | 290 |
| Fluorescence Lifetime(ns) | 28 |
| Emission Cross-section(10-18cm2)@290nm | 9.5(π), 6.1(σ) |
| Laser Threshold(μJ) | 15-25 |
| Laser Slope Efficiency | 29% |
| Estimated Pumping Efficiency | 50(π), 20(σ) |
| ESA Cross-section(10-18cm2)@266nm | 6.5π), 23(σ) |
| Gain Cross-section(10-18cm2)@290nm | 6.8(π), 1.5(σ) |
| Saturation Fluence(mJ/cm2) | 100 |
| λ(μm) | n | λ(μm) | n | λ(μm) | n |
| 0.18 | 1.51 | 0.32 | 1.45 | 5.82 | 1.39 |
| 0.19 | 1.50 | 0.43 | 1.44 | 6.20 | 1.38 |
| 0.21 | 1.49 | 0.88 | 1.43 | 6.71 | 1.37 |
| 0.22 | 1.48 | 2.67 | 1.42 | 7.00 | 1.36 |
| 0.24 | 1.47 | 3.94 | 1.41 | 7.53 | 1.35 |
| 0.27 | 1.46 | 5.01 | 1.40 | 8.22 | 1.34 |
- large band gaps and low phonon energies
- Characteristic of the Ce3+5d1–4f1 interconfigurational transition
- High fluorescence efficiencies
- Small non-linear refractive indices
- Can be pumped by the fourth harmonic of a Nd:YAG laser
- Transparency, tolerance to laser-induced damage
- Broad UVtunability (from 280 to 325 nm)
- Scintillator
- Tunable ultraviolet lasers
- Remote-sending applications
- Ultrafast pulse generation and amplification
- Power UV laser amplifiers
| [1] Mcgonigle A , Coutts D W , Girard S , et al. A 10 kHz Ce:LiSAF laser pumped by the sum-frequency-mixed output of a copper vapour laser[J]. Optics Communications, 2003, 193(1-6):233-236. |
| [2] Laroche M , Girard S , R Moncorgé, et al. Beneficial effect of Lu3+ and Yb3+ ions in UV laser materials[J]. Optical Materials, 2003, 22(2):147-154. |
| [3] Yanagida T , Kawaguchi N , Fujimoto Y , et al. Ce and Eu-doped LiSrAlF6 scintillators for neutron detectors[J]. Radiation Measurements, 2011, 46(12):1708-1711. |
| [4] Bayramian A J , Marshall C D , Wu J H , et al. Ce:LiSrAlF6 Laser Performance with Antisolarant Pump Beam[J]. Journal of Luminescence, 1996, 69(2):85-94. |
| [5] Demirbas U . Cr: Colquiriite Lasers: Current Status and Challenges for Further Progress. 2018. |
| [6] Yamaji A , Yokota Y , Yanagida T , et al. Crystal growth and dopant segregation of Ce:LiSrAlF_6 and Eu:LiSrAlF_6 crystals with high dopant concentrations[J]. Journal of Crystal Growth, 2012, 352(1):p.106-109. |
| [7] Yamaji A , Yanagida T , Kawaguchi N , et al. Crystal growth and scintillation properties of Ce and Eu doped LiSrAlF_6[J]. Nuclear Instruments & Methods in Physics Research, 2011, 659(1):p.368-372. |
| [8] Yanagida, T, Yoshikawa, et al. Crystal growth, optical properties, and a-ray responses of Ce-doped LiCaAlF6 for different Ce concentration[J]. OPTICAL MATERIALS -AMSTERDAM-, 2009. |
| [9] Yokota Y , Tanaka C , Kurosawa S , et al. Effects of Ca/Sr Ratio Control on Optical and Scintillation Properties of Eu-doped Li(Ca,Sr)AlF 6 Single Crystals[J]. Journal of Crystal Growth, 2018, 490:71-76. |
| [10] Johnson K S , Pask H M , Withford M J , et al. Efficient all-solid-state Ce:LiLuF laser source at 309 nm[J]. Optics Communications, 2005, 252(1):132-137. |
| [11] Rambaldi P , R Moncorgé, Wolf J P , et al. Efficient and stable pulsed laser operation of Ce:LiLuF 4 around 308 nm[J]. Optics Communications, 1998, 146(1-6):163-166. |
| [12] Shiran N , Gektin A , Neicheva S , et al. Energy storage in Ce-doped LiCaAlF 6 and LiSrAlF 6 crystals[J]. Radiation Measurements, 2004, 38(4-6):459-462. |
| [13] Shiran N , Gektin A , Neicheva S , et al. Energy transfer in pure and Ce-doped LiCaAlF6 and LiSrAlF6 crystals[J]. Nuclear Inst & Methods in Physics Research A, 2005, 537(1/2):266-270. |
| [14] Wakahara S , Yanagida T , Fujimoto Y , et al. Evaluation of Ce3+ and alkali metal ions Co-doped LiSrAlF6 crystalline scintillators[J]. Radiation Measurements, 2013, 56(Complete):111-115. |
| [15] Luong M V , Empizo M , Cadatal-Raduban M , et al. First-principles calculations of electronic and optical properties of LiCaAlF6 and LiSrAlF6 crystals as VUV to UV solid-state laser materials[J]. Optical Materials, 2016, 65. |
| [16] Joubert M F , Guyot Y , Jacquier B , et al. Fluoride crystals and high lying excited states of rare earth ions[J]. Journal of Fluorine Chemistry, 2001, 107(2):235-240. |
| [17] K Shimamura and H Sato and A Bensalah and H Machida and N Sarukura and T Fukuda. Growth of Ce-doped Colquiriite- and Scheelite-type single crystals for UV laser applications[J]. Optical Materials, 2002. |
| [18] Laboratory wins awards[J]. Optics & Laser Technology, 1996, 28(8):iv. |
| [19] Laser remote sensing[J]. TRENDS IN ANALYTICAL CHEMISTRY, 1998, 17(8-9):491-500. |
| [20] Samtleben T A , Hulliger J . LiCaAlF6 and LiSrAlF6: Tunable solid state laser host materials[J]. Optics and Lasers in Engineering, 2005, 43(3-5):251-262. |
| [21] Spence D J , Liu H , Coutts D W . Low-threshold miniature Ce:LiCAF lasers[J]. Optics Communications, 2006, 262(2):238-240. |
| [22] Castillo V K , Quarles G J . Progress in the crystal growth of Ce : colquiriites[J]. Journal of Crystal Growth, 1997, 174(1):337–341. |
| [23] Tiihonen M , Pasiskevicius V , F Laurell. Tailored UV-laser source for fluorescence spectroscopy of biomolecules[J]. Optics & Lasers in Engineering, 2007, 45(4):444-449. |
| [24] Fujimoto Y , Nakanishi J , Yamada T , et al. Visible fiber lasers excited by GaN laser diodes[J]. Progress in Quantum Electronics, 2013, 37(4):185-214. |
| [25] TaijuTsuboi,ValentinPetrov,FrankNoack,KiyoshiShimamura,Femtosecond relaxation in Ce ions in LiCaAlF and LiSrAlF,ScienceDirect,Volumes 323–324, 12 July 2001, Pages 688-691 |




Leave a Reply