LiCAF
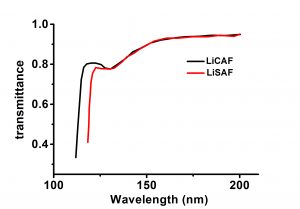
Lithium calcium hexafluoroaluminate(LiCAF) crystals are widely used as vacuum ultraviolet(VUV) and ultraviolet(UV) laser host media. It has excellent transmission characteristics down to the vacuum ultraviolet (VUV) region. The transmission edge of LiCAF was measured experimentally to be 112nm. In particular, LiCAF has a transmittance of over 95% at 157nm, which corresponds to the wavelength of an F2 laser. It was also shown that LiCAF becomes virtually non-birefringent around 152nm, thereby making it an attractive optical window material for VUV laser sources LiCAF(LiCaAIF6) are excellent laser materials with high energy storage and high slope efficiency, also ideal working material under conditions of ultra short pulse and ultra high power.
Parameter
| Orientation Tolerance | 5ˊ |
| Parallelism | <10〞 |
| Perpendicularity | 5ˊ |
| Chamfer | 0.1mm@45° |
| Surface Quality | 10/5 or better |
| Wavefront Distortion | λ/8 @632.8 nm |
| Surface Flatness | λ/10 @632.8 nm |
| Clear Aperture | >95% |
| Diameter Tolerance | +0/-0.05mm |
| Length Tolerance | ±0.1mm |
| Coatings | As per requirement |
| Damage Threshold | over 15J/cm2 TEM00, 10ns, 10Hz |
| Dopant Concentration Tolerance | 0.10% |
| Lattice | HexagonaL |
| Space Group | P31C |
| Lattice Constants | a=5.006, c=9.636Å |
| Density (g/cm3) | 2.94 |
| Melting Point | 825°C |
| Thermal Conductivity(W·m-1·K-1) | 5.14(∥a), 4.58(∥c) |
| Thermal Expansion(10-6·K-1) | 3.6(∥a), 22(∥c) |
| Specific Heat(J·g-1·K-1) | 0.935 |
| Fracture Toughness(MPa·m1/2) | 0.18~0.37 |
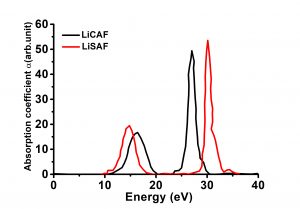
| Band Gap(eV)@LDA | 8.02(indirect) |
| Young’s Modulus(GPa) | 105.32(∥a), 94.89(∥c) |
| Bulk Modulus(GPa) | 108.01 |
| Dielectric Constant | 1.27 |
| Absorption Edge | 112nm |
| Refractive Index | no=1.3826, ne=1.3826@632,8nm |
| Thermal-optical Coefficient(10-6/°C) | -7.3(no), -4.9(ne) |
| Wavelength(nm) | no | ne |
| 632.8 | 1.3826 | 1.3826 |
| 546.1 | 1.384 | 1.384 |
| 435.8 | 1.3862 | 1.3862 |
| 253.7 | 1.4061 | 1.4073 |
- Large band gaps and low phonon energies
- Optical transmission and low thermal lensing distortion
- Absorption edge is 112nm
- Small non-linear refractive indices
- Transparency, tolerance to laser-induced damage
- Lase host media
- Lens in VUV photolithography
| [1] Tanaka C , Yokota Y , Kurosawa S , et al. Crystal growth and optical properties of indium doped LiCaAlF6 scintillator single crystals[J]. Optical Materials, 2016:S092534671630595X. |
| [2] Yoshikawa A , Yanagida T , Yokota Y , et al. Crystal growth and VUV luminescence properties of Er3+- and Tm3+-doped LiCaAlF6 for detectors[J]. Optical Materials, 2010, 32(9):845-849. |
| [3] Abdulsabirov R Y , Dubinskii M A , Korableva S L , et al. Crystal growth, EPR and site-selective laser spectroscopy of Gd 3+-activated LiCaALF 6 single crystals[J]. Journal of Luminescence, 2001, 94(none):113-117. |
| [4] Kiyoshi Shimamura and Sonia L Baldochi and Izilda M Ranieri and Hiroki Sato and Tomoyo Fujita and Vera L Mazzocchi and Carlos B.R Parente and Carlos O Paiva-Santos and Celso V Santilli and Nobuhiko Sarukura and Tsuguo Fukuda. Crystal growth of Ce-doped and undoped LiCaAlF6 by the Czochralski technique under CF4 atmosphere[J]. Journal of Crystal Growth, 2001. |
| [5] Ford M A , BE O’Day, Mcclory J W , et al. Development of a neutron spectrometer utilizing rubberized Eu:LiCAF wafers[J]. Nuclear Instruments & Methods in Physics Research, 2019. |
| [6] Yoshikawa A , Iguchi T , Boulon G , et al. Development of novel rare earth doped fluoride and oxide scintillators for two-dimensional imaging[J]. Journal of Rare Earths, 2011, 29(012):1178-1182. |
| [7] Gaabel K M , Russbuldt P , Lebert R , et al. Diode pumped Cr^3^+:LiCAF fs-laser[J]. OPTICS COMMUNICATIONS, 1998. |
| [8] Fasoli M , Vedda A , Moretti F , et al. Effect of Eu and Pb doping on the dosimetric properties of LiCAF[J]. Radiation Measurements, 2010, 45(3-6):556-558. |
| [9] Tanaka C , Yokota Y , Kurosawa S , et al. Effects of Na co-doping on optical and scintillation properties of Eu:LiCaAlF6 scintillator single crystals[J]. Journal of Crystal Growth, 2016:S0022024816306984. |
| [10] A M A F , A B E O , A M C , et al. Evaluation of Eu:LiCAF for neutron detection utilizing SiPMs and portable electronics[J]. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 2018, 908:110-116. |
| [11] Luong M V , Empizo M , Cadatal-Raduban M , et al. First-principles calculations of electronic and optical properties of LiCaAlF6 and LiSrAlF6 crystals as VUV to UV solid-state laser materials[J]. Optical Materials, 2016, 65. |
| [12] Sato H , Machida H , Nikl M , et al. Growth and characterization of aliovalent ion-doped LiCaAlF 6 single crystals[J]. Journal of Crystal Growth, 2003, 250(1):83-89. |
| [13] Shimamura K , Na M , Nakano K . Growth and Characterization of Ce-Doped LiCaAlF6 Single Crystals[J]. Journal of Crystal Growth, 1999, 197(4):896-900. |
| [14] Tanaka C , Yokota Y , Kurosawa S , et al. Growth and radioluminescence of metal elements doped LiCaAlF6 single crystals for neutron scintillator[J]. Radiation Measurements, 2016:170-173. |
| [15] Shimamura K , Baldochi S L , Na M , et al. Growth of Ce-doped LiCaAlF6 and LiSrAlF6 single crystals by the Czochralski technique under CF4 atmosphere[J]. Journal of Crystal Growth, 2000, 211(1/4):302-307. |
| [16] Dk A , Gla B , Pr A . Growth of Cr:LiCaAlF 6 and Cr:LiSrAlF 6 by the Czochralski method[J]. Journal of Crystal Growth, 2000, 210( 4):683-693. |
| [17] Sato H , Bensalah A , Yoshikawa A , et al. Improvement in the quality of LiCaAlF 6 single crystal as window material[J]. Optical Materials, 2003, 24(1-2):123-127. |
| [18] Vazquez R M , Santos M T , Lopez F J . Influence of neutral environment in the growth of Cr-doped LiCAF/LiSAF crystals: X-ray powder diffraction and EPR analysis[J]. Journal of Crystal Growth, 2002, s 237–239:894-898. |
| [19] Samtleben T A , Hulliger J . LiCaAlF6 and LiSrAlF6: Tunable solid state laser host materials[J]. Optics and Lasers in Engineering, 2005, 43(3-5):251-262. |
| [20] Stanciu M , Grattan K . LiCAF crystal-based optical fiber thermometry[J]. Sensors & Actuators A Physical, 2002, 99(3):277-283. |
| [21] Castillo V K , Chang R . Material and laser characterizations of intermediate compositions of Ce:LiSrxCa1-xAlF6[J]. Journal of Crystal Growth, 2001, 225(2-4):445-448. |
| [22] Futami, Kurosawa, Watanabe,等. Neutron detection with LiCaAlF_6 scintillator doped with 3d-transition metal ions. |
| [23] Kozeki T , Suzuki Y , Sakai M . Observation of new excitation channel of cerium ion through highly vacuum ultraviolet transparent LiCAF host crystal[J]. Journal of Crystal Growth, 2001, 229:501-504. |
| [24] Kole M , Chauvin M , Fukazawa Y , et al. PoGOLino: A scintillator-based balloon-borne neutron detector[J]. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 2015. |
| [25] Castillo V K , Quarles G J . Progress in the crystal growth of Ce : colquiriites[J]. Journal of Crystal Growth, 1997, 174(1):337–341. |
| [26] Gvishi R , Gonen E , Kalisky Y . Studies of the spectroscopic behavior of Cr+3:LiCAF pumped by a solid-state dye laser[J]. Optical Materials, 1999, 13(1):129-133. |
| [27] Yokota, Yuui, Yoshikawa,等. Temperature-dependent evaluation of Nd:LiCAF optical properties as potential vacuum ultraviolet laser material. |
| [28] Sato H , Bensalah A , Solovieva N , et al. X-ray damage characterization in BaLiF3,KMgF3 and LiCaAlF6 complex fluorides[J]. Radiation Measurements, 2004, 38(4-6):463-466. |
| [29] BirsenAyaz-Maierhafer,Carl G.Britt,Andrew J.August,HairongQi,Carolyn E.Seifert,Jason P.Hayward,Design optimization for a wearable, gamma-ray and neutron sensitive, detector array with directionality estimation,ScienceDirect,Volume 870, 21 October 2017, Pages 131-139 |





Leave a Reply