LaCl3
LaCl3 crystal has UCl3 type structure, space group P63/m, and it has attracted much attention for its high light output as well as good energy resolution. These unique properties made LaCl3 crystal a promising material as scintillator in the field of high-energy physics experiments and medical imaging, as well. LaCl3 (doped with 10% Ce3+) has a very high light output (49,000 photons/MeV), and fast principle decay time constant (26 ns) . These properties make LaCl3:Ce a very promising material for gamma-ray spectroscopy.
LaCl3 crystal belongs to hexagonal system, density is 3.8g/cm3. Its energy resolution is 3.1%, decay time With 26 ns and a time resolution of 224 ps, there is almost no damage after exposure to gamma rays up to 3 kGy. Such excellent scintillation performance is very rare in inorganic compounds. The energy is 60 keV to 1275 keV. Under the excitation of γ-ray source, the nonlinear response coefficient of light output is 7%, which is far superior to LSO:Ce crystal (35%), NaI: Tl crystal (15%) and CsI: Tl (20%). Based on its good numbers, this scintillation material can find its place in such applications as medical imaging, nuclear physics, X-ray diffraction, non-destructive evaluation, treaty verification and safeguards, environmental monitoring, and geological exploration.
Parameter
| Material | LaCl3 |
| Appearance | white odorless powder |
| Crystal structure | hexagonal (UCl3 type), hP8 |
| Space group | P63/m, No. 176 |
| Lattice constant | a = 0.74779 nm, b = 0.74779 nm, c = 0.43745 nm |
| Formula units (Z) | 2 |
| Coordination geometry | Tricapped trigonal prismatic,(nine-coordinate) |
| Density (g/cm3) | 3.8 |
| Melting point | 860 °C |
| Boiling point | 1,000 °C |
| Solubility in water | 957 g/L (25 °C) |
| Emission peak (nm) | 350, 430 |
| Decay time (ns) | 28 |
| Energy resolution R (%) | 10.5 ± 0.9 |
| Photon yield (103 ph/MeV) | 34 ± 1 |
| Light yield (photons/keV) | 49 |
| Light output (photons/MeV) | 50,500 |
| Absorbed γ-ray energy (keV) | 662, 60 |
| Photoelectron yield [% of NaI(TI)](for γ-rays) | 35 |
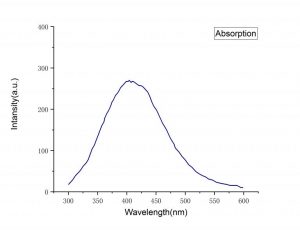 | 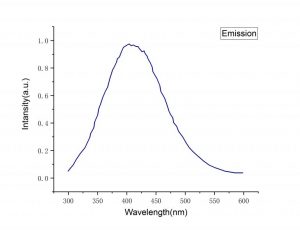 |
| LaCl3 Absorption Spectra | LaCl3 Emission Spectra |
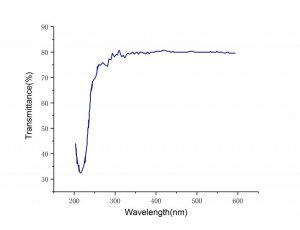 | 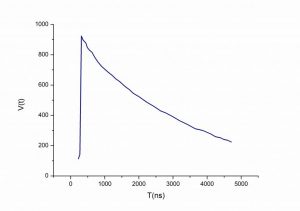 |
| LaCl3 Transmission Spectra | LaCl3 Decay Time |
- Excellent energy resolution
- Good time resolution
- High chemical resistance
- Fast decay times – 28nsec
- High light outputs – 49,000 Photons/MeV
- Optical outputs with good linearity with temperature
- Excellent radiation hardness
- Safety inspection
- Geological exploration
- Environmental testing
- Medical – SPECT
- Industrial – Well logging
- Nuclear and high energy physics – specialist applications
| [1] Hongbing, Chen, and, et al. Bridgman growth of LaCl3:Ce3+ crystal in non-vacuum atmosphere[J]. Journal of Alloys and Compounds, 2008. |
| [2] Balcerzyk M , Moszynski M , Kapusta M . Comparison of LaCl3:Ce and NaI(Tl) scintillators in g-ray spectrometry[J]. NUCLEAR INSTRUMENTS AND METHODS IN PHYSICS RESEARCH SECTION A, 2005. |
| [3] Tai S , Bo Z , Hui W , et al. Dehydrogenation properties of LaCl3 catalyzed NaAlH4 complex hydrides[J]. Journal of Alloys & Compounds, 2009, 467(1-2):413-416. |
| [4] Sahoo D K , Mishra R , Singh H , et al. Determination of thermodynamic stability of lanthanum chloride hydrates (LaCl3⋅xH2O) by dynamic transpiration method[J]. Journal of Thermal Analysis & Calorimetry, 2014, 588(3):578-584. |
| [5] Zheng X , Ping L , Humail I S , et al. Effect of catalyst LaCl3 on hydrogen storage properties of lithium alanate (LiAlH4)[J]. International Journal of Hydrogen Energy, 2007, 32(18):4957-4960. |
| [6] Hai-liang, Deng, Jin-huang, et al. Ablation and oxidation resistance properties of C/C composites densified by xylene pyrocarbon using LaCl3 as a catalyst and resin carbon – ScienceDirect[J]. Carbon, 144:842-842. |
| [7] Yuan X Z , Pan G , Chen H , et al. Phosphorus fixation in lake sediments using LaCl3-modified clays[J]. Ecological Engineering, 2009, 35(35):1599-1602. |
| [8] 佚名. Proton response of CEPA4: A novel LaBr3(Ce)-LaCl3(Ce) phoswich array for high-energy gamma and proton spectroscopy[J]. Nuclear Instruments and Methods in Physics Research, Section A. Accelerators, Spectrometers, Detectors and Associated Equipment, 2015, 769:105-111. |
| [9] Babiano V , Caballero L , Calvo D , et al. γ-Ray position reconstruction in large monolithic LaCl_3(Ce) crystals with SiPM readout[J]. Nuclear instruments and methods in physics research, 2019, 931(JUL.1):1-22. |
| [10] Yeung Y Y , Tanner P A . New analyses of energy level datasets for LaCl3:Ln3+ (Ln = Pr, Nd, Er)[J]. Journal of Alloys & Compounds, 2013, 575:54-60. |
| [11] Naqvi A A , Al-Matouq F A , Khiari F Z , et al. Sample dependent response of a LaCl3:Ce detector in prompt gamma neutron activation analysis of bulk hydrocarbon samples[J]. Nuclear Inst & Methods in Physics Research A, 2013, 719(aug.11):39-43. |
| [12] Krupa P . Strong and weak up-conversion rate in LaCl3: U3+ single crystal[J]. Journal of Alloys and Compounds, 2004. |
| [13] Bernabei R , Belli P , F Montecchia, et al. Performances and potentialities of a LaCl3:Ce scintillator[J]. Nuclear Instruments & Methods in Physics Research, 2005, 555(1/2):270-281. |
| [14] Elvira, Peringer, and, et al. On the synthesis of LaCl3 catalysts for oxidative chlorination of methane[J]. Applied Catalysis A: General, 2008, 350(2):178-185. |
| [15] Meng D , Zhao Q , X Pan, et al. Preparation of La2O3 by ion-exchange membrane electrolysis of LaCl3 aqueous solution[J]. Journal of Rare Earths, 2019. |
| [16] Tarannum S , Ahmed N , Siddiqui Z N . LaCl3/nano-SiO2: A novel nanocatalyst for efficient synthesis of functionalized 2,3-dihydroquinazolinones[J]. Catalysis Communications, 2015, 66:60-66. |
| [17] Yu, Pei, and, et al. Growth and luminescence characteristics of undoped LaCl3 crystal by Modified Bridgman Method – ScienceDirect[J]. Journal of Crystal Growth, 2005, 279(3-4):390-393. |
| [18] Effects of LaCl_3 on the growth and photosynthetic characteristics of Fny-infected tobacco seedlings[J]. Journal of Rare Earths, 2012. |
| [19] LaCl3 Flux mediated Ta3N5 Planar Photoanode for Solar Water Oxidation[J]. Chemical Engineering Journal, 2020, 396:125161. |
| [20] Zhou X , Wang Y . LaCl3-modified Ni deposits on 3D-heterotypic porous Ti surface for strengthening its mechanical and electrochemical properties[J]. Surface and Coatings Technology, 2019, 375. |
| [21] Iwadate Y , Suzuki K , Onda N , et al. Local structure of molten LaCl3 analyzed by X-ray diffraction and La-LIII absorption-edge XAFS technique[J]. Journal of Alloys & Compounds, 2006, 408(none):248-252. |
| [22] O. Tengblad and T. Nilsson and E. Nácher and H.T. Johansson and J.A. Briz and M. Carmona-Gallardo and C. Cruz and V. Gugliermina and A. Perea and J. Sanchez del Rio and M. Turrión Nieves and J. Bergström and E. Blomberg and A. Bülling and E. Gallneby and J. Hagdahl and L. Jansson and K. Jareteg and R. Masgren and M. Nordström and G. Risting and S. Shojaee and H. Wittler. LaBr3(Ce):LaCl3(Ce) Phoswich with pulse shape analysis for high energy gamma-ray and proton identification[J]. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 2013. |
| [23] Xl Z , Ocka-Malicka M , Szczepaniak W . Internal cation mobility in molten KClLaCl 3[J]. Journal of Molecular Liquids, 1999. |
| [24] Yin M , Joubert M F , Krupa J C . Infrared to green up-conversion in LaCl$_3$:U$^{3+}$[J]. Journal of Luminescence, 1997, 75(3):221-227. |
| [25] Gao D . Influence of LaCl3 addition on microstructure and properties of nickel-electroplating coating[J]. Journal of Rare Earths, 2013. |
| [26] Mclntyre J I , Schrom B T , Cooper M W , et al. LaCl 3:Ce coincidence signatures to calibrate gamma-ray detectors[J]. Nuclear Instruments & Methods in Physics Research, 2011, 652(1):201-204. |
| [27] Taggart M P , Henderson J . Fast-neutron response of LaBr3(Ce) and LaCl3(Ce) scintillators[J]. Nuclear Instruments and Methods in Physics Research Section A Accelerators Spectrometers Detectors and Associated Equipment, 2020, 975:164201. |
| [28] 周洁, 郭兰萍, 张霁, et al. Effects of LaCl3 on photosynthesis and the accumulation of tanshinones and salvianolic acids in Salvia miltiorrhiza seedlings[J]. Journal of Rare Earths, 2011, 29(5):494-498. |



Leave a Reply