LiSAF
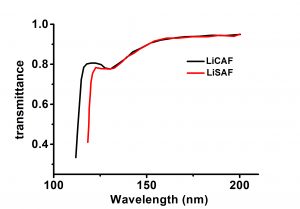
Lithium strontium hexafluoroaluminate(LiSAF) crystals are widely used as vacuum ultraviolet(VUV) and ultraviolet(UV) laser host media. It has excellent transmission characteristics down to the vacuum ultraviolet (VUV) region. The transmission edge of LiSAF was measured experimentally to be 116nm. LiSAF(LiSrAIF6) are excellent laser materials with high energy storage and high slope efficiency, also ideal working material under conditions of ultra short pulse and ultra high power. Like Ti:sapphire, LiSAF exhibits a broad bandwidth, tunable from 760 to 1000 nm. Unlike Ti:sapphire, however, it has a relatively long upper-state lifetime (60µs) and, therefore, can be flashlamp-pumped. This advantage, coupled with the fact that LiSAF has a low nonlinear refractive index and low thermal lensing, make it an ideal candidate for high-power short pulse laser systems.
Parameter
| Orientation Tolerance | 5ˊ |
| Parallelism | <10〞 |
| Perpendicularity | 5ˊ |
| Chamfer | 0.1mm@45° |
| Surface Quality | 10/5 or better |
| Wavefront Distortion | λ/8 @632.8 nm |
| Surface Flatness | λ/10 @632.8 nm |
| Clear Aperture | >95% |
| Diameter Tolerance | +0/-0.05mm |
| Length Tolerance | ±0.1mm |
| Coatings | As per requirement |
| Damage Threshold | over 15J/cm2 TEM00, 10ns, 10Hz |
| Dopant Concentration Tolerance | 0.10% |
| Lattice | HexagonaL |
| Space Group | P31C |
| Lattice Constants | a=5.08, c=10.214Å |
| Density (g/cm3) | 3.45 |
| Melting Point | 750°C |
| Thermal Conductivity(W·m-1·K-1) | 3.09(∥a), 4.58(∥c) |
| Thermal Expansion(10-6·K-1) | -10(∥a), 18.8(∥c) |
| Specific Heat(J·g-1·K-1) | 0.842 |
| Fracture Toughness(MPa·m1/2) | 0.4 |
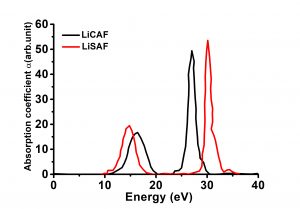
| Band Gap(eV)@LDA | 7.92(indirect) |
| Young’s Modulus(GPa) | 86.33(∥a), 83.45(∥c) |
| Bulk Modulus(GPa) | 83.75 |
| Dielectric Constant | 1.26 |
| Absorption Edge | 116nm |
| Refractive Index | no=1.3944, ne=1.3988@632,8nm |
| Thermal-optical Coefficient(10-6/°C) | -4.5(no), -9.1(ne) |
| Wavelength(nm) | no | ne |
| 632.8 | 1.3944 | 1.3988 |
| 546.1 | 1.3972 | 1.4011 |
| 435.8 | 1.4014 | 1.4052 |
| 253.7 | 1.422 | 1.4276 |
- Large band gaps and low phonon energies
- Optical transmission and low thermal lensing distortion
- Absorption edge is 116nm
- Small non-linear refractive indices
- Transparency, tolerance to laser-induced damage
- Lase host media
- Lens in VUV photolithography
| [1] Vazquez R M , Santos M T , Lopez F J . Influence of neutral environment in the growth of Cr-doped LiCAF/LiSAF crystals: X-ray powder diffraction and EPR analysis[J]. Journal of Crystal Growth, 2002, s 237–239:894-898. |
| [2] Vasa N J , Parhat H , Okada T , et al. Performance of an optical fiber butt-coupled Cr3+:LiSrAlF6 laser[J]. Optics Communications, 1998, 147(1-3):196-202. |
| [3] Eichenholz J M , Richardson M , Mizell G . Diode pumped, frequency doubled LiSAF microlaser[J]. Optics Communications, 1998, 153(4-6):263-266. |
| [4] Luong M V , Empizo M , Cadatal-Raduban M , et al. First-principles calculations of electronic and optical properties of LiCaAlF6 and LiSrAlF6 crystals as VUV to UV solid-state laser materials[J]. Optical Materials, 2016, 65. |
| [5] Samtleben T A , Hulliger J . LiCaAlF6 and LiSrAlF6: Tunable solid state laser host materials[J]. Optics and Lasers in Engineering, 2005, 43(3-5):251-262. |
| [6] Wakahara S , Yanagida T , Fujimoto Y , et al. Evaluation of Ce3+ and alkali metal ions Co-doped LiSrAlF6 crystalline scintillators[J]. Radiation Measurements, 2013, 56(Complete):111-115. |
| [7] Suzuki S , Yokota Y , Yamaji A , et al. Effects of Eu concentration control on crystal growth and scintillation properties for Eu:LiSrAlF6 crystals[J]. Optical Materials, 2014, 36(12):1959-1962. |
| [8] Yamaji A , Yanagida T , Kawaguchi N , et al. Crystal growth and scintillation properties of Ce and Eu doped LiSrAlF_6[J]. Nuclear Instruments & Methods in Physics Research, 2011, 659(1):p.368-372. |
| [9] Yanagida T , Kawaguchi N , Fujimoto Y , et al. Ce and Eu-doped LiSrAlF6 scintillators for neutron detectors[J]. Radiation Measurements, 2011, 46(12):1708-1711. |
| [10] True M , Kirm M , Negodine E , et al. VUV spectroscopy of Tm and Mn doped LiSrAlF. 2004. |
| [11] Castillo V K , Quarles G J . Progress in the crystal growth of Ce : colquiriites[J]. Journal of Crystal Growth, 1997, 174(1):337–341. |
| [12] Castillo V K , Chang R . Material and laser characterizations of intermediate compositions of Ce:LiSrxCa1-xAlF6[J]. Journal of Crystal Growth, 2001, 225(2-4):445-448. |
| [13] Shimamura K , Baldochi S L , Na M , et al. Growth of Ce-doped LiCaAlF6 and LiSrAlF6 single crystals by the Czochralski technique under CF4 atmosphere[J]. Journal of Crystal Growth, 2000, 211(1/4):302-307. |
| [14] Dk A , Gla B , Pr A . Growth of Cr:LiCaAlF 6 and Cr:LiSrAlF 6 by the Czochralski method[J]. Journal of Crystal Growth, 2000, 210( 4):683-693. |
| [15] Yamaji A , Yokota Y , Yanagida T , et al. Crystal growth and dopant segregation of Ce:LiSrAlF_6 and Eu:LiSrAlF_6 crystals with high dopant concentrations[J]. Journal of Crystal Growth, 2012, 352(1):p.106-109. |
| [16] Bayramian A J , Marshall C D , Wu J H , et al. Ce:LiSrAlF6 Laser Performance with Antisolarant Pump Beam[J]. Journal of Luminescence, 1996, 69(2):85-94. |
| [17] M. H . Chemical stability and Ce doping of LiMgAlF6 neutron scintillator[J]. Journal of Alloys and Compounds: An Interdisciplinary Journal of Materials Science and Solid-state Chemistry and Physics, 2015. |
| [18] Abdulsabirov R Y , Dubinskii M A , Korableva S L , et al. Crystal growth, EPR and site-selective laser spectroscopy of Gd 3+-activated LiCaALF 6 single crystals[J]. Journal of Luminescence, 2001, 94(none):113-117. |
| [19] Jomar B. Amaral and Mario E.G. Valerio and Marcos A. Couto dos Santos and Robert A. Jackson. Defect simulation and crystal field studies of Ln3+:LiCaAlF6 and LiSrAlF6[J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 2004. |
| [20] Sutherland J M , Ruan S , Mellish R , et al. Diode-pumped, single-frequency, Cr:LiSAF coupled-cavity microchip laser[J]. Optics Communications, 1995, 113( 4–6):458-462. |
| [21] Yokota Y , Tanaka C , Kurosawa S , et al. Effects of Ca/Sr Ratio Control on Optical and Scintillation Properties of Eu-doped Li(Ca,Sr)AlF 6 Single Crystals[J]. Journal of Crystal Growth, 2018, 490:71-76. |
| [22] Shiran N , Gektin A , Neicheva S , et al. Energy storage in Ce-doped LiCaAlF 6 and LiSrAlF 6 crystals[J]. Radiation Measurements, 2004, 38(4-6):459-462. |
| [23] Shiran N , Gektin A , Neicheva S , et al. Energy transfer in pure and Ce-doped LiCaAlF6 and LiSrAlF6 crystals[J]. Nuclear Inst & Methods in Physics Research A, 2005, 537(1/2):266-270. |
| [24] Nicholls J . Laser performance and spectroscopic properties of Nd3+-doped LiKYF5 and LiKGdF5 crystals[J]. Optics Communications, 1997, 137(s 4–6):281–284. |
| [25] Backer A D , Garreau J C , Razdobreev I M , et al. Laser performance of new crystal Cr3+:(CeGd)Sc3(BO3)4[J]. Optics Communications, 2003, 222(1):351-354. |
| [26] Kozeki T , Suzuki Y , Sakai M . Observation of new excitation channel of cerium ion through highly vacuum ultraviolet transparent LiCAF host crystal[J]. Journal of Crystal Growth, 2001, 229:501-504. |
| [27] Passilly N , Haouas E , V Ménard, et al. Population lensing effect in Cr:LiSAF probed by Z-scan technique[J]. Optics Communications, 2006, 260(2):703-707. |
| [28] Satoru Kuze and Douglas du Boulay and Nobuo Ishizawa and Nobuhiro Kodama and Mitsuo Yamaga and Brian Henderson. Structures of LiCaAlF6 and LiSrAlF6 at 120 and 300 K by synchrotron X-ray single-crystal diffraction[J]. Journal of Solid State Chemistry, 2004. |
| [29] Ruiz M , Barbosa E A , Maldonado E P , et al. Zone melting growth of LiSrAlF6:Cr crystals for diode laser pumping[J]. Journal of Crystal Growth, 2002, 241(1-2):177-182. |
| [30] Long X , Lin Z , Hu Z , et al. Optical study of Cr3+-doped LaSc3(BO3)4 crystal[J]. Journal of Alloys & Compounds, 2002, 347(s 1–2):52-55. |



Leave a Reply