CaF2
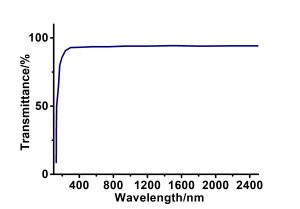
Calcium fluoride(CaF2) is a very important optical functional crystal, which has good optical properties, mechanical properties and chemical stability. It can be used as optical crystal, laser crystal and inorganic scintillation crystal. It has a wide transmittance range (0.125-10 μm), and is widely used as optical medium from vacuum ultraviolet to mid infrared. CaF2 crystal is an ideal optical material for achromatic lenses because of its special refractive index and relative dispersion. At present, deep UV excimer laser lithography is developing from 193 nm to 121 nm. CaF2 single crystal has the advantages of good ultraviolet transmittance, high laser damage resistance threshold, low stress birefringence and high refractive index, which makes it the best choice for the research of deep UV excimer laser lithography. In terms of laser applications, laser diode pumped CaF2 crystals activated by Er3+, Tm3+, Yb3+ and other 3-valent rare earth ions were obtained at room temperature.
Parameter
| Orientation | [100] or [001] < ±0.5° |
| Orientation Tolerance | < 0.5° |
| Parallelism | <20〞 |
| Perpendicularity | 5ˊ |
| Surface Quality | 10-5 (MIL-O-13830A) |
| Wavefront Distortion | <λ/4@633 nm |
| Surface Flatness | <λ/8 @633 nm |
| Clear Aperture | >90% |
| Thickness/Diameter Tolerance | ±0.05 mm |
| Crystal System | Isometric |
| Habit | Cubic, massive |
| Space Group | Fm3m |
| Class (H-M) | m3m (4/m 3 2/m) – Hexoctahedral |
| Lattice Constants | 5.4626 Å |
| Z | 4 |
| Streak | White |
| Density | 3.175…3.56g/cm3 |
| Melting Point | 1418°C |
| Thermal Conductivity /(W·m-1·K-1@25°C) | 9.71@[111] |
| Heat Capacity/(J ·(kg·K)-1) | 854 |
| Thermal Expansion /(10-6·K-1@25°C ) | 18.9 |
| Young`s Modulus /GPa | 75.8 |
| Tenacity | Brittle |
| Cleavage Quality | Perfect, perfect and easy |
| Hardness (Mohs) | 4 |
| Fracture | Conchoidal |
| Transmission Range | 0.125 – 10 µm |
| Refractive Index | 1.432-1.436 |
| Reflective Loss | 2.89@4µm |
| Optical Character | Isotropic |
| Surface Relief | Moderate |
| Birefringence | None |
| Pleochroism | Absent |
| Dispersion | 0.007 |
| Poisson Ratio | 0.29 |
| Dielectric Constant | 6.76 |
| λ(μm) | n | λ(μm) | n | λ(μm) | n |
| 0.18 | 1.51 | 0.32 | 1.45 | 5.82 | 1.39 |
| 0.19 | 1.50 | 0.43 | 1.44 | 6.20 | 1.38 |
| 0.21 | 1.49 | 0.88 | 1.43 | 6.71 | 1.37 |
| 0.22 | 1.48 | 2.67 | 1.42 | 7.00 | 1.36 |
| 0.24 | 1.47 | 3.94 | 1.41 | 7.53 | 1.35 |
| 0.27 | 1.46 | 5.01 | 1.40 | 8.22 | 1.34 |
- Excellent transmission from 125nm to 10um
- Good optical properties, mechanical behavior and chemical stability
- Anisotropy, soft and brittle, high coefficient of thermal expansion and easy cleavage
- Special refractive index and relative dispersion value
- Soluble in acids
- Good ultraviolet transmittance, high laser damage resistance threshold, low stress birefringence and high refractive index
- Optical crystal, laser crystal(IR, UV, VUV)
- Optical media and optical material
- Best material for the study of deep UV excimer laser lithography
- Substrate, optical waveguide
- IR analytic, astronomical-und camera lenses and optics for Excimer laser
| [1] Kacyuba A V , Dvurechenskii A V , Kamaev G N , et al. Radiation-Induced epitaxial CaSi2 film growth at the molecular-beam epitaxy of CaF2 on Si[J]. Materials Letters, 2020, 268:127554-. |
| [2] Mir A , Zaoui A , Bensaid D . The displacement effect of a fluorine atom in CaF2 on the band structure[J]. Applied Surface Science, 2018, 439(MAY1):1180-1185. |
| [3] Ha A , Rla B , No C , et al. Swift heavy ion irradiation to non-amorphizable CaF2 and amorphizable Y3Al5O12 (YAG) crystals – ScienceDirect[J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 2020, 474:78-82. |
| [4] Yang L , Hinsberg V V . Liquid immiscibility in the CaF2-granite system and trace element partitioning between the immiscible liquids[J]. Chemical Geology, 2019. |
| [5] CC Lopes, Barros V , Asfora V K , et al. Optically Stimulated Luminescence of CaF 2 :Ce[J]. Journal of Luminescence, 2018:S0022231317320823. |
| [6] Shtan’Ko V , Chinkov E , Shrayber A , et al. Intrinsic luminescence of CaF2 crystals under simultaneous excitation of pulsed accelerated electrons and stimulated emission of semiconductor CdSSe[J]. Radiation Physics and Chemistry, 2019, 168:108619. |
| [7] Sun J L , Jie J C , Zou Q C , et al. Boron removal from molten silicon using CaO-SiO_2-BaO-CaF_2 slag. 中国有色金属学报(英文版), 2016. |
| [8] Fangfang H U , Zhao Z , Chi F , et al. Structural characterization and temperature-dependent luminescence of CaF 2 :Tb 3+ /Eu 3+ glass ceramics[J]. Journal of Rare Earths, 2017, 35(6):536-541. |
| [9] Kato T , Nakauchi D , Kawaguchi N , et al. Radio-photoluminescence phenomenon in non-doped CaF2 ceramic[J]. Materials Letters, 2020, 270:127688. |
| [10] Batool A , Izerrouken M , Aisida S O , et al. Effect of Ca colloids on in-situ ionoluminescence of CaF2 single crystals[J]. Nuclear Instruments and Methods in Physics Research Section B Beam Interactions with Materials and Atoms, 2020, 476:40-43. |
| [11] [, Min, Li, et al. The combined effect of CaF2 coating and La-doping on electrochemical performance of layered lithium-rich cathode material[J]. Electrochimica Acta, 2018. |
| [12] Song L , Gao J , Wang X , et al. Highly luminescent Cit/CaF2: Ce3+, Tb3+ nanoparticles and detection of Cu2+ ions[J]. Journal of Luminescence, 2020, 226:117445. |
| [13] Xu C , Flodstr?M K , Esmaeilzadeh S . Low temperature densification of B4C ceramics with CaF2/Y2O3 additives[J]. International Journal of Refractory Metals & Hard Materials, 2012, 35(none):311-314. |
| [14] Li W , Jing W , Mei B , et al. Effect of NaF doping on the transparency, microstructure and spectral properties of Yb3+: CaF2 transparent ceramics[J]. Journal of the European Ceramic Society, 2020, 40(13). |
| [15] Guo J , Gong J , Shi P , et al. Study on the polishing mechanism of pH-dependent tribochemical removal in CMP of CaF 2 crystal[J]. Tribology International, 150. |
| [16] Kawaguchi N , Kimura H , Takebuchi Y , et al. Dosimetric properties of non-doped LiF/CaF2 eutectic[J]. Radiation Measurements, 2020, 132. |
| [17] Li W , Huang H , Mei B , et al. Fabrication, microstructure and laser performance of Yb3+ doped CaF2-YF3 transparent ceramics[J]. Ceramics International, 2020, 46(11). |
| [18] Ji L , Chen Z , Guo R , et al. Preparation of nano – coating powder CaF2@Al(OH)3 and its application in Al2O3/Ti(C,N)self-lubricating ceramic tool materials[J]. Ceramics International, 2020, 46(10). |
| [19] Liu L , Song J , Li W , et al. Effect of sintering temperature on the microstructure and optical properties of Mn: CaF 2 transparent ceramics[J]. Materials Chemistry and Physics, 2017:S0254058417308520. |
| [20] Tan W , Yy A , Xja C , et al. A new method to synthesize Sub-10 nm CaF 2 : Nd 3+ nanoparticles and fluorescent enhancement via Li + ions or Ce 3+ ions doping[J]. Dyes and Pigments, 175. |
| [21] [, Mayur, A, et al. Performance assessment of CaF2 solid lubricant assisted minimum quantity lubrication in turning[J]. Procedia Manufacturing, 2019. |
| [22] Xm A , Xh A , Lei L A , et al. Crystallisation kinetics and structural stability of transparent CaF2 glass ceramics: Dependence of light transmittance on the amount of CaF2 added – ScienceDirect[J]. Ceramics International, 2020, 46( 10):15314-15324. |
| [23] Khan W N , Chhibber R . Physicochemical and thermo physical characterization of CaO–CaF 2 –SiO 2 and CaO–TiO 2 –SiO 2 based electrode coating for offshore welds[J]. Ceramics International, 2020, 46( 7):8601-8614. |
| [24] Azzato G , Marco G D , Stellato V , et al. Tortuosity and connectivity evaluation by CFD simulation for morphological characterization of membranes and catalytic structures. Case study: CaF2-like structure[J]. Chemical Engineering Science, 2018. |
| [25] Vgi A , Sysa B , Mgzb C , et al. Multimodal upconversion CaF2:Mn/Yb/Er/Si nanoparticles – ScienceDirect[J]. Journal of Fluorine Chemistry, 231. |
| [26] Chuklina N , Mysovsky A . Theoretical study of self-trapped hole diffusion in CaF2, SrF2, BaF2 crystals[J]. Radiation Measurements, 2019, 128:106135-. |
| [27] Miao X , Bai Z , Huo X , et al. Controllable preparation of CaF2 transparent glass ceramics: Dependence of the light transmittance mechanism on the glass crystallization behaviour[J]. Ceramics International, 2019. |
| [28] Gong J , Xiao C , Yu J , et al. Stress-enhanced dissolution and delamination wear of crystal CaF2 in water condition[J]. WEAR -LAUSANNE-, 2019. |
| [29] Dai S , Yan G , Wang L , et al. Enhanced electrochemical performance and thermal properties of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material via CaF2 coating[J]. Journal of electroanalytical chemistry, 2019, 847:113197. |
| [30] Kawano N , Kawaguchi N , Fukuda K , et al. Scintillation and dosimetric properties of Ce-doped ~6LiF-CaF_2 eutectic composites[J]. Optical Materials, 2018, 82(Aug.):60-64. |





Leave a Reply