BaF2
Barium fluoride (BaF2) is a general-purpose optical window material that offers a wide range of transparency, from the ultraviolet to the long-wave infrared, with low reflectance loss and low dispersion. BaF2 single crystal is an intensively studied scintillator for the detection of gamma radiation due to its relatively high stopping power, radiation hardness and extremely fast response. BaF2 possesses the fast cross-luminescence component at 195 and 220 nm with a lifetime of several hundred picoseconds. However, this component coexists with slow one at 310 nm related to the self-trapped exciton (STE). BaF2 is a broad band gap crystal with Eg=10:9 eV. It is currently regarded as the fastest inorganic scintillator which has cross-luminescence bands peaked at 195 and 220 nm and a broad band peaking at about 300 nm due to self-trapped excitons. Much attention has been focused on the luminescent properties of rare earth ions activated BaF2.
Parameter
| Orientation | [100] or [111] < ±0.5° |
| Angle Tolerance | < 0.5° |
| Parallelism | <20〞 |
| Perpendicularity | 5ˊ |
| Surface Quality | 10-5 (MIL-O-13830A) |
| Wavefront Distortion | <λ/4@633 nm |
| Surface Flatness | <λ/8 @633 nm |
| Clear Aperture | >90% |
| Thickness/Diameter Tolerance | ±0.05 mm |
| Crystal System | Isometric |
| Habit | Cubic |
| Space Group | Fm3m(Oh5 |
| Lattice Constants | 6.2001 Å |
| Specific mass | 4.886 g/cm3 |
| Melting Point | 1354°C |
| Flexure Strength (MPa) | 27 |
| Tenacity | Brittle |
| Thermal Conductivity /(W·cm-1·K-1@25°C) | 0.07 |
| Specific Heat/ (J·g-1·K-1) | 1.003 |
| Thermal Expansion /(10-6·K-1@25°C ) | 13.7 |
| Hardness (kg/mm2@Knoop) | 78 |
| Young`s Modulus /GPa | 138.5 |
| Fracture | {111} and {100} |
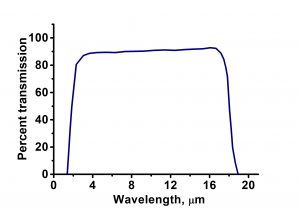
| Transmission Range | 0.15 … 12 µm |
| Reflective Loss | 6 … 16%@0.2 … 10 µm |
| Thermo-optic coefficient(10-6·K-1@30…90°C ) | -18.8 |
| Poisson Ratio | 0.31 |
| Dielectric Constant | 7.33@f=2MHz |
| λ(μm) | n | λ(μm) | n | λ(μm) | n |
| 0.2 | 1.5573 | 4 | 1.4558 | 9 | 1.4144 |
| 0.5 | 1.4779 | 5 | 1.4511 | 10 | 1.4011 |
| 1 | 1.4686 | 6 | 1.4441 | 11 | 1.3865 |
| 2 | 14647 | 7 | 1.4357 | 12 | 1.3696 |
| 3 | 1.4612 | 8 | 1.4258 | 12.5 | 1.3585 |
| 15 | 1.305 |
- Excellent transmission from 150nm to 12um
- Chemically stable
- Low reflectance loss and low dispersion
- High stopping power, radiation hardness and extremely fast response
- Broad band gap
- Susceptible to thermal shock
- Possesses the fast cross-luminescence component at 195 and 220 nm with a lifetime of several hundred picoseconds
- Window and focusing mirror for deep uv and excimer lasers
- Scintillator,IR optics
- Inorganic scintillator for subnanosecond timing
| [1] Ilves V G , SY Sokovnin, Zuev M G , et al. Study of d0 magnetism of BaF2 nanopowder after thermal and radiation exposure[J]. Journal of Magnetism and Magnetic Materials, 2020:166666. |
| [2] Takumi, Kato, Go, et al. Development of BaF2 transparent ceramics and evaluation of the scintillation properties[J]. Radiation Measurements, 2017. |
| [3] Lesniak M , Mach G , Starzyk B , et al. Investigation of the structure in oxyfluoride TeO2–P2O5 based glasses with the various BaF2 content[J]. Journal of Molecular Structure, 2020, 1217:128452. |
| [4] Stef M , Nicoara I , Racu A , et al. Spectroscopic properties of the gamma irradiated ErF3-DOPED BaF2 crystals[J]. Radiation Physics and Chemistry, 2020:109024. |
| [5] Novotny R . Performance of the BaF2-calorimeter TAPS 1[J]. Nuclear Physics B – Proceedings Supplements, 1998, 61(3):137–142. |
| [6] Khg A , Msb A , Apj A , et al. TL properties of BaF 2 :Ce phosphor for high gamma ray dosimetry[J]. Journal of Luminescence, 2019, 209:316-320. |
| [7] Chuklina N , Mysovsky A . Theoretical study of self-trapped hole diffusion in CaF2, SrF2, BaF2 crystals[J]. Radiation Measurements, 2019, 128:106135-. |
| [8] M Chylii, T Malyi, I Rovetskyi,等. Diffusion of 5p-holes in BaF2 nanoparticles[J]. Optical Materials, 2019, 91(MAY):115-119. |
| [9] Radzhabov E A , Kozlovsky V A . Electron transfer between heterogeneous lanthanides in BaF2 crystals[J]. Radiation Measurements, 2019. |
| [10] Antonyak O T , Vistovsk Yy V V , Zhyshkovych A V , et al. Size effects and radiation resistance of BaF2 nanophosphors[J]. Journal of Luminescence, 2019, 211. |
| [11] He W , Zheng W , Xie P , et al. Compositional correlation and polymorphism in BaF_2-PrF_3 thin films deposited using electron-beam evaporation[J]. Thin Solid Films, 2019, 669(JAN.1):558-563. |
| [12] Adams S , Tan E S . Pathways for ion transport in nanostructured BaF 2:CaF 2[J]. Solid State Ionics, 2008, 179(1):33-37. |
| [13] Thomas M E , Tropf W J . Barium Fluoride (BaF 2 )[M]. 1997. |
| [14] Mendoza E , Cano-Ott D , Guerrero C , et al. Pulse pile-up and dead time corrections for digitized signals from a BaF2 calorimeter[J]. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 2014, 768:55-61. |
| [15] Zhao X , You H , Gao F . DNA-assisted rational design of BaF_2 linear and erythrocyteshaped nanocrystals[J]. 中国化学工程学报(英文版), 2018. |
| [16] Shang Y D , Zhang J L , Yan W , et al. Molecular simulation investigation on the interaction between barrier-to-autointegration factor dimer or its Gly25Glu mutant and LEM domain of emerin[J]. Computational Biology & Chemistry, 2014, 53(pt.b):184-190. |
| [17] Long R , Luo J , Chen M , et al. Oxidative coupling of methane over BaF2-promoted rare earth oxides with variable valence[J]. Applied Catalysis A General, 1997, 159(1):171-185. |
| [18] Jia S , Li C , Zhao Z , et al. Er 3+ -doped ZnF 2 -BaF 2 -SrF 2 -YF 3 fluoride glasses for 2.7 μm laser applications[J]. Materials Letters, 2018, 227. |
| [19] Yang X L , Wang W C , Zhang Q Y . BaF 2 modified Cr 3+ /Ho 3+ co-doped germanate glass for efficient 2.0 μm fiber lasers[J]. Journal of Non-Crystalline Solids, 2017, 482:147-153. |
| [20] El-Moneim A , Amin. BaF 2 –contained tellurite glasses: Quantitative analysis and prediction of elastic properties and ultrasonic attenuation–Part I[J]. Journal of Fluorine Chemistry, 2018, 210:156-165. |
| [21] Sun X , Yang Q , Xie P , et al. Effects of substitution of BaF2 for GdF3 on optical properties of dense oxyfluoride borogermanate scintillating glasses[J]. Journal of Rare Earths, 2015. |
| [22] Zhao, Yongpeng, Zhang, et al. Formation of nanostructures induced by capillary-discharge soft X-ray laser on BaF2 surfaces[J]. Applied Surface Science: A Journal Devoted to the Properties of Interfaces in Relation to the Synthesis and Behaviour of Materials, 2017, 396(Feb.28 Pt.2):1201-1205. |
| [23] Marczewska A , Roda M , M Nocuń. Thermal and spectroscopic characterization of gallium-tellurite glasses doped BaF2 and PbO[J]. JOURNAL OF NONCRYSTALLINE SOLIDS, 2017, 464(1):104-114. |
| [24] Qy A , Bc A , Yao Z B , et al. Impact of gas-water ratios on N 2 O emissions in biological aerated filters and analysis of N 2 O emissions pathways[J]. Science of The Total Environment, 723. |
| [25] Okada G , Shinozaki K , Komatsu T , et al. Radio-photoluminescence in Sm-doped BaF2-Al2O3-B2O3 glass-ceramics[J]. Radiation Measurements, 2016, 106. |
| [26] Ye L , Peng X , Zhang S , et al. Photoluminescence properties of Ca-doped BaMgAl10O17:Eu2+,Mn2+ blue phosphor using BaF2 and CaF2 as co-flux[J]. Journal of Rare Earths, 2014, 32(12):1109-1113. |
| [27] Murakami T , Ouyang J H , Sasaki S , et al. High-temperature tribological properties of Al 2O 3, Ni–20 mass% Cr and NiAl spark-plasma-sintered composites containing BaF 2–CaF 2 phase[J]. Wear, 2005, 259(1):626-633. |
| [28] Kanai K , Fukui Y , Kozawa T , et al. Effect of BaF2 powder addition on the synthesis of YAG phosphor by mechanical method[J]. Advanced Powder Technology, 2017, 28(1):50-54. |
| [29] Shi H , Wang Y , Ran J , et al. Ab initio calculations of the hydroxyl impurities in BaF2[J]. Computational Materials Science, 2011, 50(11):3101-3104. |
| [30] Balabhadra S , Reid M F , V Golovko, et al. Absorption Spectra, Defect Site Distribution and Upconversion Excitation Spectra of CaF$_2$/SrF$_2$/BaF$_2$:Yb$^{3+}$:Er$^{3+}$ Nanoparticles[J]. 2019. |






Leave a Reply