LiI
Lithium iodide is white crystalline solid salt but when exposed in air it becomes yellow in color as oxidation of iodine to iodine takes place. Lithium iodide is extensively used as an electrolyte for high temperature batteries. It is also used for providing longer life to the batteries. Furthermore, lithium iodide is soluble in propanol, ethanol, ethanediol and ammonia.
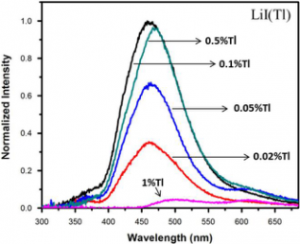
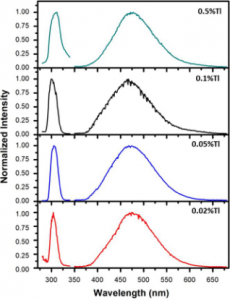
Alkali halide scintillators are among the popular materials for scintillation applications and are widely used in the areas such as high energy physics, nuclear physics, medicine, geology, astrophysics and security. LiI crystals have attracted attention owing to the presence of 6Li nuclei that have a large capture cross-section for thermal neutrons. LiI crystals have been grown with different activators and tested for scintillation properties.
Parameter
| Orientation | <100>, <110>, <111> |
| Orientation Tolerance | < 0.5° |
| Parallelism | 5〞 |
| Perpendicularity | 3ˊ |
| Surface Quality | 10-5 (Scratch/Dig) |
| Wavefront Distortion | <λ/4@632 nm |
| Surface Flatness | <λ/8 @632 nm |
| Clear Aperture | >90% |
| Chamfer | <0.1×45° |
| Thickness/Diameter Tolerance | ±0.05 mm |
| Crystal Structure | Cubic |
| Symmetry Class | Fm3m |
| Lattice Constants | 5.93 |
| Specific Mass | 4.076 g/cm3 |
| Melting Point | 449°C |
| Cleavability | <100> |
| Thermal Conductivity /(W·m-1·K-1@300K) | 0.702 |
| Specific Heat (J·kg-1·K-1) | 258.12 |
| Thermal Expansion(10-6·K-1) | 40 |
| Hardness (Mohs) | 2 |
| Vickers Microhardness(GPa) | 0.1 |
| Elastic Constant(kbar)@295K | C11=290.7, C12=-142.1, C44=140.7 |
| Bulk Modulus (GPa) | 17.17 |
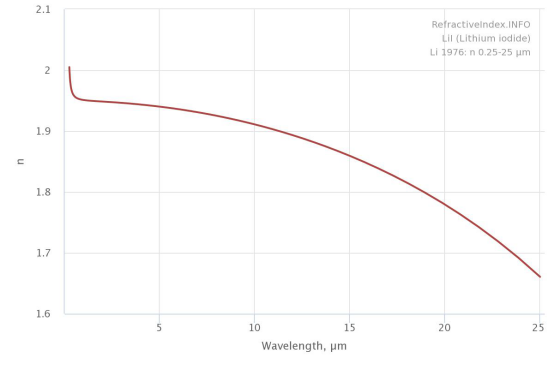
| Refractive Index | 1.955 |
| 6LiI(Eu) | 6LiI(Tl) | |
| Maximun emmission(nm) | 470 | 460 |
| Energy Resolution at ∆E/E(FWHM)% | 13@0.1mole% | |
| Light Yield(ph/MeV) | 15000 | 14000@0.5mole% |
| Primary Decay Time(μs) | 1.2 | 0.177(81%)@0.1mole% |
- The presence of 6Li nuclei
- Have a large capture cross-section for thermal neutrons
- Scintillation
- Thermal neutronsdetector
- High count rate applications
| [1] Jkba B , Hwka B , Ga A , et al. Study on LiI and KI with low melting temperature for electrolyte replenishment in molten carbonate fuel cells[J]. International Journal of Hydrogen Energy, 2019, 44( 47):25930-25938. |
| [2] Ryutaro S . Probing the solid–liquid transition of thin propanol and butanol films through interactions with LiI[J]. Chemical Physics Letters, 2018, 706:393-399. |
| [3] Optimization of crystal growth and properties of γ-CuI ultrafast scintillator by the addition of LiI[J]. Materials Research Bulletin, 2018, 106(OCT.):228-233. |
| [4] [, Motoshi, Suyama,等. Lithium dissolution/deposition behavior with Li3PS4-LiI electrolyte for all-solid-state batteries operating at high temperatures[J]. Electrochimica Acta, 2018. |
| [5] Yu H , Lu H , Hu X , et al. LiI-KI and LAGP electrolytes with a bismuth-tin positive electrode for the development of a liquid lithium battery[J]. Materials Chemistry and Physics, 2020, 247:122865-. |
| [6] Asfand F , Stiriba Y , Bourouis M . Performance evaluation of membrane-based absorbers employing H2O/(LiBr + LiI + LiNO3 + LiCl) and H2O/(LiNO3 + KNO3 + NaNO3) as working pairs in absorption cooling systems[J]. Energy, 2016, 115(pt.1):781-790. |
| [7] Kim J , D Shin, Jung Y , et al. LiCl-LiI molten salt electrolyte with bismuth-lead positive electrode for liquid metal battery[J]. JOURNAL OF POWER SOURCES, 2018, 377(FEB.15):87-92. |
| [8] Wu Z , Xu J , Zhang Q , et al. LiI embedded meso-micro porous carbon polyhedrons for lithium iodine battery with superior lithium storage properties[J]. Energy Storage Materials, 2017:S240582971730257X. |
| [9] Fan B , Fu H , Li H , et al. Ionic conductive GeS2-Ga2S3-Li2S-LiI glass powders prepared by mechanical synthesis[J]. Journal of Alloys & Compounds, 2018:61-67. |
| [10] Kamada K , Chiba H , Yoshino M , et al. Growth and scintillation properties of Eu doped LiSrI 3 /LiI eutectics[J]. Optical Materials, 2017:S0925346717302951. |
| [11] Zl A , Yao Z A , Jh A , et al. Fast lithium ionic conductivity observed in LiI-MoS2 composite – ScienceDirect[J]. Inorganic Chemistry Communications, 112. |
| [12] Simonsson J , Olofsson N E , Hosseinnia A , et al. Influence of potassium chloride and other metal salts on soot formation studied using imaging LII and ELS, and TEM techniques[J]. Combustion & Flame, 2018, 190(APR.):188-200. |
| [13] Miyazaki R , Hihara T . Fabrication of LiI-LiBH4 solid solutions by cryomilling[J]. Materials Letters, 2020, 271. |
| [14] Gupta R K , Bedja I , Islam A , et al. Electrical, structural, and thermal properties of succinonitrile-LiI-I2 redox-mediator[J]. SOLID STATE IONICS, 2018. |
| [15] Li S F , Zhang Y , Huang N , et al. Daphnane diterpenoids from the stems of Trigonostemon lii and their anti-HIV-1 activity[J]. Phytochemistry, 2013. |
| [16] Yu Z , Li H , Li K , et al. A series of LiI/acetamide phase transition electrolytes and their applications in dye-sensitized solar cells[J]. ELECTROCHIMICA ACTA, 2010. |
| [17] Liu Z , Pu X , Fei G , et al. Atomistic Insights into the Reaction Mechanism of Nanostructured LiI: Implications for Rechargeable Li-I 2 Batteries[J]. Energy Storage Materials, 2018:S2405829718306974-. |
| [18] Rajagopal R , Ryu K S . Facile synthesis of 3Li2S-P2S5:xLiCl:(1-x)LiI glass ceramic solid electrolyte for solid-state lithium-ion batteries[J]. Journal of Alloys and Compounds, 2019, 798:235-242. |
| [19] A L X , B L G B , C M G L , et al. A new bird track, Koreanaornis lii ichnosp. nov. from the Lower Cretaceous Hekou Group in the Lanzhou-Minhe Basin, Gansu, Northwest China, and implications for Early Cretaceous avian diversity[J]. Cretaceous Research, 2016, 66:141-154. |
| [20] Spannenberger S , Mi V , Klotz E , et al. Annealing-induced vacancy formation enables extraordinarily high Li+ ion conductivity in the amorphous electrolyte 0.33 LiI+ 0.67 Li3PS4[J]. Solid State Ionics, 2019, 341:115040. |
| [21] Kartini E , Nakamura M , Arai M , et al. Structure and dynamics of solid electrolyte (LiI)0.3(LiPO3)0.7[J]. Solid State Ionics, 2014, 262:833-836. |
| [22] Takekawa R , Kawamura J . Study of ion dynamics of LiI·6H2O in the supercooled liquid state using NMR spectroscopy and ionic conductivity measurements[J]. Chemical Physics, 2020, 536:110815. |
| [23] Sarnacki B G , Chelliah H K . Sooting limits of non-premixed counterflow ethylene/oxygen/inert flames using LII: Effects of flow strain rate and pressure (up to 30atm)[J]. Combustion & Flame, 2018:S0010218018301391. |
| [24] Wang J , Deng M , Chen Y , et al. Structural, elastic, electronic and optical properties of lithium halides (LiF, LiCl, LiBr, and LiI): First-principle calculations[J]. Materials Chemistry and Physics, 2020, 244:122733. |
| [25] A D J D , A H J K , B S K , et al. Thermoluminescence kinetic features of Lithium Iodide (LiI) single crystal grown by vertical Bridgman technique – ScienceDirect[J]. Optical Materials, 2017, 70:120-126. |
| [26] Fp A , Jjc A , Iv A , et al. Soot primary particle sizing in a n-heptane doped methane/air laminar coflow diffusion flame by planar two-color TiRe-LII and TEM image analysis[J]. Fuel, 266. |
| [27] Leverick G , Tulodziecki M , Tatara R , et al. Solvent-Dependent Oxidizing Power of LiI Redox Couples for Li-O2 Batteries[J]. Joule, 2019, 3(4):1106-1126. |
| [28] Cheng X , Chen L , Yan F . Study of the characteristic of diesel spray combustion and soot formation using laser-induced incandescence (LII)[J]. Journal- Energy Institute, 2014, 87(4):383-392. |
| [29] Nagata H , Chikusa Y , Akimoto J . A high rate performance positive composite electrode using a high P/S ratio and LiI composite solid electrolyte for an all-solid-state Li–S battery[J]. Journal of Power Sources, 2020, 453(4):227905. |




Leave a Reply