LiF
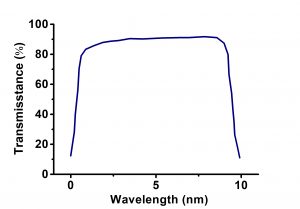
Lithium fluoride (LiF) crystal has excellent VUV region transmittance. It is used for windows, prisms, and lenses in the visible and infrared in 0.104μm-7μm. LiF single crystal is sensitive to thermal shock and would be attacked by atmospheric moisture at 400°C. In addition irradiation produces color centers. Modest precautions should be taken against moisture and high energy radiation damage. Besides LiF softens at 600°C and is slightly plastic that can be bent into radius plates. The material can be cleaved along (100) plane and less commonly (110) plane. The optical characteristics are good and yet the structure is not perfect and cleavage is difficult. For good structure LiF is less commonly grown by the Kyropoulos method (air-grown) specifically for monochromator plates. High quality LiF is usually grown by modified Bridgman method.
Parameter
| Orientation | [100]< ±0.5° |
| Angle Tolerance | < 0.5° |
| Parallelism | <20〞 |
| Perpendicularity | 5ˊ |
| Surface Quality | 10-5 (MIL-O-13830A) |
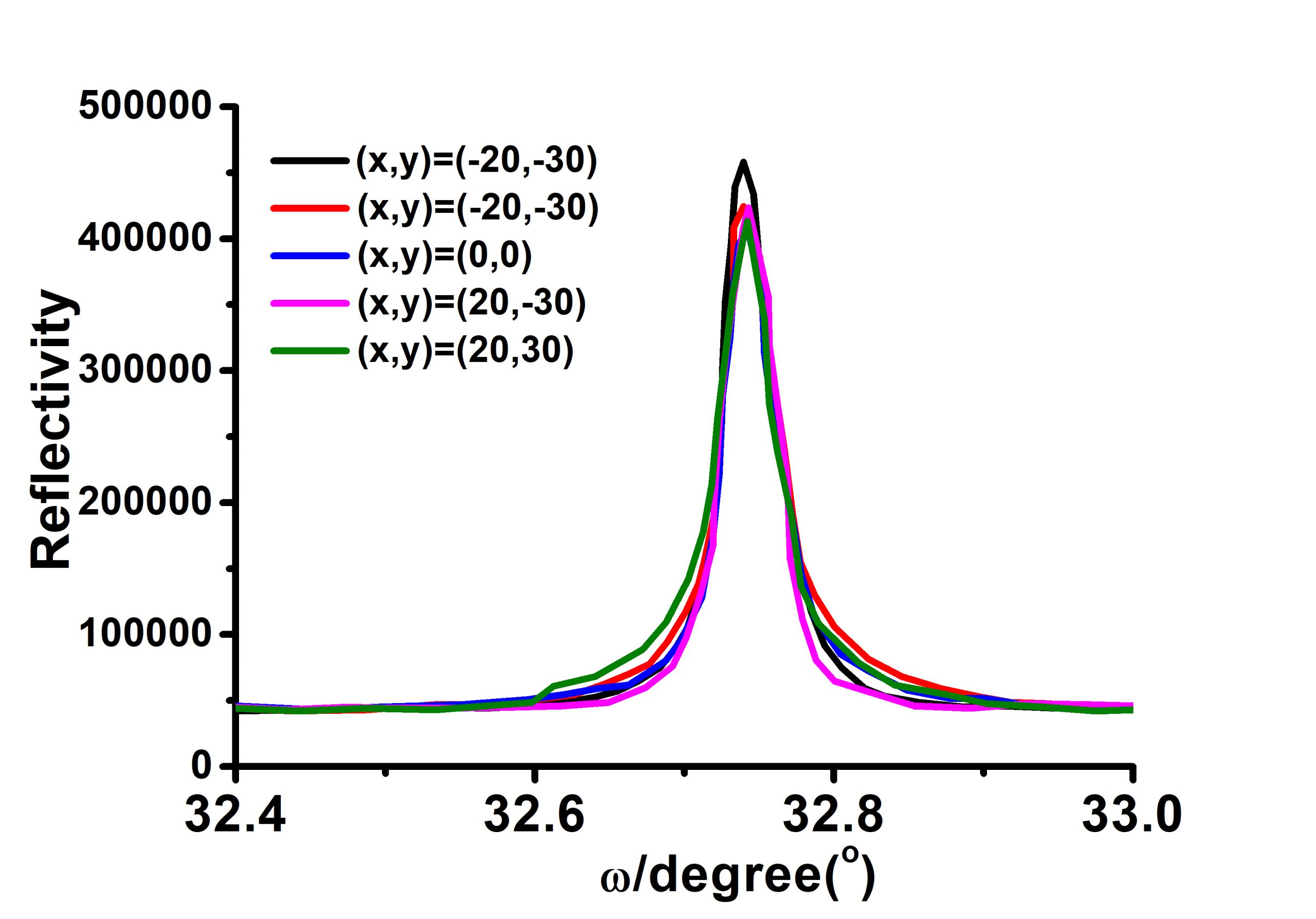
| Wavefront Distortion | <λ/4@633 nm |
| Surface Flatness | <λ/8 @633 nm |
| Clear Aperture | >90% |
| Chamfer | Technological for protection from |
| edge cracks | |
| Thickness/Diameter Tolerance | ±0.05 mm |
| Crystal Structure | Cubic (NaCl) |
| Lattice Constants | 4.026 |
| Density | 2.639 g/cm3 |
| Melting Point | 870°C |
| Thermal Conductivity /(W·m-1·K-1@314k) | 4.01 |
| Specific Heat Capacity/ (J·kg-1·K-1) | 1562 |
| Thermal Expansion /(10-6·K-1@283k ) | 37 |
| Hardness (Knoop) | 102-113@600g |
| Young`s Modulus /(1011dyne cm-2) | 6.5-7.6 |
| Elastic Coefficient | C11=112; C12=46; C44= 63.5 |
| Apparent Elastic Limit/ MPa | 11.2@1620 psi |
| Cleavage | -100 |
| Transmission Range | 0.105 … 6 µm |
| Refractive Index | no = 1.37327@2.5µm, 1.624@0.12 µm |
| Reflective Loss | 4.4% @4.0 µm |
| Poisson Ratio | 0.326 |
| Absorption Coefficient/cm-`1 | 0.74×10-3@2.7µm |
- Tends to create color centers
- Max temperature for application:400°C
- Excellent VUV region transmittance
- Sensitive to thermal shock
- X-ray monochromator plates
- Optical material for VUV applications
- Windows, prisms, and lenses
| [1] Li Z , Li R , Li X , et al. LIF in embryo culture medium is a predictive marker for clinical pregnancy following IVF-ET of patients with fallopian tube obstruction[J]. Journal of Reproductive Immunology, 2020, 141:103164. |
| [2] Xu R , Zhou Y , Shi L , et al. In-situ preparation of LixSn-Li2O-LiF/reduced graphene oxide composite anode material with large capacity and high initial Coulombic efficiency[J]. Journal of Power Sources, 2020. |
| [3] Hu C , Zhang P , Wang X , et al. Improvement of fluidization quality of a LiF bed using internal blades[J]. Powder Technology, 2020, 362:817-825. |
| [4] Zhang P , Zhen G , Yang M . A novel high-Q and low-temperature sintering Li2Mg3Ti1-x(Al1/2Nb1/2)xO6-4wt%LiF microwave dielectric ceramics[J]. Materials Chemistry and Physics, 2020, 250:123134. |
| [5] Zbf A , Bjt A , Wfw A , et al. Sintering behavior and microwave dielectric properties of Li 4 Mg 3 [Ti 0.8 (Mg 1/3 Ta 2/3 ) 0.2 ] 2 O 9 ceramics with LiF additive for LTCC applications[J]. Journal of Alloys and Compounds, 822. |
| [6] Tan D , X Zhang, X Liu, et al. Stability enhancement of inverted perovskite solar cells using LiF in electron transport layer[J]. Organic Electronics, 2019, 80:105613. |
| [7] Yang X A , Qzc A , Cwz B . Liquid phase in-situ synthesis of LiF coated boron powder composite and performance study – ScienceDirect[J]. Defence Technology, 2020, 16( 3):635-641. |
| [8] Batool A , Aisida S O , Hussain J , et al. In-situ investigation of point defects kinetics in LiF using ion luminescence technique[J]. Nuclear Instruments and Methods in Physics Research Section B Beam Interactions with Materials and Atoms, 2020, 466:52-55. |
| [9] Azizi-Malekabadi M , Sarraf-Mamoory R . Devising a novel method of producing high transparent magnesium aluminate spinel (MgAl 2 O 4 ) ceramics body using synthesized LiF nanopowder and spark plasma sintering[J]. Materials Chemistry and Physics, 250. |
| [10] Tk A , Wk B , Yy C , et al. Characterisation and comparison of micropatterns written using a proton beam in Ag-activated glass and LiF crystal observed by single- and multi-photon microscopy – ScienceDirect[J]. Radiation Measurements, 134. |
| [11] Lu N , He G , Yang Z , et al. Fabrication and densification mechanism of MgO/Graphene composites with LiF as additive[J]. Scripta Materialia, 2020, 174:91-94. |
| [12] Zhu X , Sun S , Sun T , et al. Electrical conductivity of REF3-LiF (RE=La and Nd) molten salts[J]. Journal of Rare Earths, 2019, 38(6). |
| [13] Bilski P , Marczewska B , Gieszczyk W , et al. Fluorescent imaging of heavy charged particle tracks with LiF single crystals[J]. Journal of Luminescence, 2019, 213. |
| [14] Kolek P , Andrzejak M , Uchacz T , et al. Consistent Franck-Condon Modeling of Geometry Changes for the S0→S1(ππ*) Excitation in Anthranilic Acid: LIF Spectroscopy Aided by CC2 or TDDFT Vibrations[J]. Journal of Quantitative Spectroscopy and Radiative Transfer, 2019, 242:106747. |
| [15] Yu Z , Zhang J , Wang C , et al. Flame-retardant concentrated electrolyte enabling a LiF-rich solid electrolyte interface to improve cycle performance of wide-temperature lithium-sulfur batteries[J]. Journal of Energy Chemistry, 2020, 51:154-160. |
| [16] Abbasalizadeh A , Sridar S , Chen Z , et al. Experimental investigation and thermodynamic modelling of LiF-NdF3-DyF3 system[J]. Journal of Alloys & Compounds, 2018:S0925838818312933. |
| [17] Bilski P , Marczewska B , Gieszczyk W , et al. Luminescent properties of LiF crystals for fluorescent imaging of nuclear particles tracks[J]. Optical Materials, 2019, 90(APR.):1-6. |
| [18] Zhang Y , Liu P , Li Y , et al. Study on fluorescence spectroscopy of PAHs with different molecular structures using laser-induced fluorescence (LIF) measurement and TD-DFT calculation[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2020, 224:117450-. |
| [19] Su M M . COVID-19 lockdown: de-risking exit by protecting the lung with leukaemia inhibitory factor (LIF)[J]. Medicine in Drug Discovery, 6. |
| [20] Yuan Y , Wu F , Chen G , et al. Porous LiF layer fabricated by a facile chemical method toward dendrite-free lithium metal anode[J]. 能源化学:英文版, 2019, 028(010):P.197-203. |
| [21] Nghia N V , Hieu H K , Duc N B , et al. Investigation of pressure effects on melting temperature and shear modulus of B1-LiF[J]. Chemical Physics, 2020, 538:110862. |
| [22] Kawaguchi N , Kimura H , Takebuchi Y , et al. Dosimetric properties of non-doped LiF/CaF2 eutectic[J]. Radiation Measurements, 2020, 132. |
| [23] Zhang P , Wu S , Zhao Y , et al. Effects of LiF on sintering characteristics and dielectric properties of low-loss SrCuSi 4 O 10 ceramics for LTCC applications[J]. Materials Chemistry and Physics, 2019, 222:246-250. |
| [24] Li Z , Cao K , Li J , et al. Modification of interface between PEDOT:PSS and perovskite film inserting an ultrathin LiF layer for enhancing efficiency of perovskite light-emitting diodes[J]. Organic Electronics, 2020, 81:105675. |
| [25] A Windmüller, Dellen C , Lobe S , et al. Thermal stability of 5V LiCoMnO 4 spinels with LiF additive[J]. Solid State Ionics, 2018, 320:378-386. |
| [26] Ma H J , Kong J H , Kim D K . Insight into the scavenger effect of LiF on extinction of a carboxylate group for mid-infrared transparent Y2O3MgO nanocomposite[J]. Scripta Materialia, 2020, 187:37-42. |
| [27] M Tański, Kocik M , Hrycak B , et al. Measurement of OH radicals distribution in a microwave plasma sheet using LIF method[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2019, 222:117268-. |
| [28] Margioula-Siarkou C , Prapas Y , Petousis S , et al. LIF endometrial expression is impaired in women with unexplained infertility while LIF-R expression in all infertility sub-groups[J]. Cytokine, 2017, 96:166-172. |
| [29] Christiansson M , Bernhardsson C , Geber-Bergstrand T , et al. OSL in NaCl vs. TL in LiF for absorbed dose measurements and radiation quality assessment in the photon energy range 20keV to 1.3MeV[J]. Radiation Measurements, 2018, 112:11-15. |
| [30] The solid electrolytes Li2O–LiF–Li2WO4–B2O3 with enhanced ionic conductivity for lithium-ion battery |





Leave a Reply