YLiF4
YLF laser crystal is the abbreviation for yttrium lithium fluoride (YLiF4). YLF laser crystal is birefringent, which eliminates thermally induced depolarization loss. Pure YLF crystals are transparent within the spectrum band of 0.12 – 7.5 µm, photo-, thermal- and radiation-resistant. The YLF crystals have low values of nonlinear refraction index and thermos optical constants. YLF has good optical properties with high transparency throughout the emission spectrum of the conventional sources used for pumping solid-state lasers. YLF does not show UV damage, and it has lower non-radiative decay rates for processes occurring between electronic levels participating in the pumping and lasing process. YLF is also a good medium for mode locking at 1047 or 1053 nm and 1.313 µm as a result of its natural birefringence and low thermal lensing. Mode-locked pulses from YLF are shorter thanks to its broader linewidth, both for the 1047/1053-nm and 1.313-µm emission peaks. Yttrium Lithium Fluoride (YLF) is a crystalline material used in optics and solid-state single crystal laser rods. YLF is typically doped with materials such as neodymium, holmium, erbium and is generally immediately available in most volumes.
Parameter
| Doping Concentration | 0.5–3.0% |
| Orientation Tolerance | 5ˊ |
| Dopant Concentration Tolerance | 0.10% |
| Parallelism | <10〞 |
| Perpendicularity | 5ˊ |
| Chamfer | 0.1mm@45° |
| Surface Quality | 10-5 (MIL-O-13830A) |
| Wavefront Distortion | <λ/8@633 nm |
| Surface Flatness | λ/10 @633 nm |
| Clear Aperture | 95% |
| Diameter | 2-50.8 mm |
| Length | 1-180mm |
| Crystal System | Scheelite Structure |
| Symmetry | Tetragonal |
| Space Group | C64h-T41/a |
| Lattice Constants | a=5.1710, c=10.7484 Å |
| Density (g/cm3) | 3.96 |
| Melting Point | 1354°C |
| clearage | Typical |
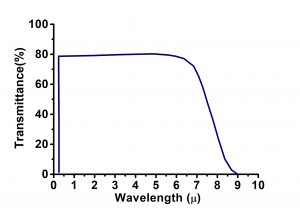
| Transmission Range | 0.22 … 8 µm |
| Reflective Loss | 6 … 16%@0.2 … 10 µm |
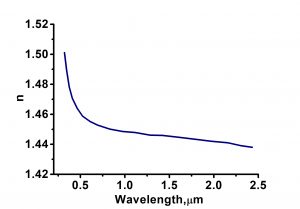
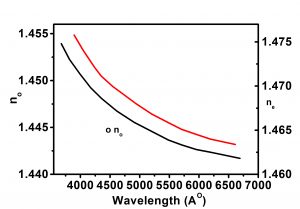
| Refractive Index | no=1.46136, ne=1.48398@435.8nm |
- High power, low beam divergence, efficient single mode operation.
- High average power Q-switched at a moderate repetition rate
- Linear polarized resonators for Q-switching and frequency doubling
- Potential uniform mode for large diameter rods or slabs
- Lamp pumping, diode pumping
- Conventional sources used for pumping solid-state lasers
- Cascade emission between intermediate levels as well as an up-converter
- A good medium for mode locking at 1047 or 1053 nm and 1.313 µm
| [1] 王殿元, 郭艳艳. Analysis and direct calculation of the energy migration interaction parameter in high concentration Nd^3+ doped YAG laser crystal[J]. 中国稀土学报:英文版, 2009(1):62-65. |
| [2] Littleford T E , Jackson R A , Read M . An atomistic surface simulation study predicting morphologies and segregation in yttrium lithium fluoride[J]. Surface Science, 2012, 606(19-20):1550-1555. |
| [3] Burlot R , R Moncorgé, Boulon G . Visible and infrared luminescence properties of Er 3+ -doped Sc 2 : O 3 : LiNbO 3 crystal fibers[J]. Journal of Luminescence, 1997, s 72–74(96):135-138. |
| [4] Hai G , Min Y , Zhang W . Upconversion of Er 3+ Ions in LiKGdF 5: Er 3+, Dy 3+ Single Crystal Produced by Infrared and Green Laser[J]. Journal of Rare Earths, 2006, 24(006):740-744. |
| [5] Bensalah A , Guyot Y , Brenier A , et al. Spectroscopic Properties of Yb3+:LuLiF4 Crystal Grown by the Czochralski Method for Laser Applications and Evaluation of Quenching Processes: A Comparison with Yb3+:YLiF4.[J]. Cheminform, 2005, 36(3). |
| [6] Bikhert Y V , Lisitsyn V M , Lisitsyna L A , et al. Pulsed cathodoluminescence of YLiF4 crystals at 15 K[J]. Nuclear Inst & Methods in Physics Research B, 2014, 327:121-123. |
| [7] Monte A , Messias D N . Photon migration at multiple frequencies in YLiF4:Nd3+ crystal[J]. Journal of Luminescence, 2010, 130(4):674-677. |
| [8] Martin J , Boonyarith T , Manson N B . Optical/RF double resonances in YLiF 4 : Ho 3+[J]. Journal of Luminescence, 1995, 63(5):297-300. |
| [9] Suzuki T , Masaki S I , Mizuno K , et al. Preparation of novel transparent glass–ceramics containing fluoride crystals[J]. Optical Materials, 2011, 33( 12):1943-1947. |
| [10] Yanagida T , Fujimoto Y , Ishizu S , et al. Optical and scintillation properties of Nd differently doped YLiF4 from VUV to NIR wavelengths[J]. Optical Materials, 2015, 41:36-40. |
| [11] Triboulet A , Rogalski A . Materials science and applications of solid crystals[J]. III-Vs Review, 2001, 14(2):46-50. |
| [12] Manoj, Kumar, Mahata, et al. Host Sensitized Luminescence and Time-Resolved Spectroscopy of YVO4: Ho3+ Nanocrystals[J]. Physics Procedia, 2015. |
| [13] Chen D , Wang Y , Yunlong Y U , et al. Infrared to ultraviolet upconversion luminescence in Nd 3+ doped nano-glass-ceramic[J]. Journal of Rare Earths, 2008, 26( 3):428-432. |
| [14] En De B , Brooks R L , Tiedje H F , et al. Measurement of the 4F5/2 and 2H(2)9/2 manifold lifetime in Nd3+:YLiF4[J]. Journal of Luminescence, 2006, 117(1):13-19. |
| [15] Bensalah A , Shimamura K , Sudesh V . Growth of Tm, Ho-codoped YLiF4 and LuLiF4 single crystals for eye-safe lasers[J]. Journal of Crystal Growth, 2001, 223(4):539-544. |
| [16] Nakauchi D , Okada G , Koshimizu M , et al. Optical and scintillation properties of Nd-doped SrAl2O4 crystals[J]. Journal of Rare Earths, 2016, 34(8):757-762. |
| [17] Bensalah A , Guyot Y , Ito M , et al. Growth of Yb 3+-doped YLiF 4 laser crystal by the Czochralski method. Attempt of Yb 3+ energy level assignment and estimation of the laser potentiality[J]. Optical Materials, 2004, 26(4):375-383. |
| [18] Cheng J , Wen J , Chen Y , et al. Crystal-field analyses for trivalent lanthanide ions in LiYF4[J]. Journal of Rare Earths, 2016, 34(10):1048-1052. |
| [19] J López-Solano, P Rodríguez-Hernández, Mu?Oz A , et al. Theoretical study of the scheelite-to-fergusonite phase transition in YLiF 4 under pressure[J]. Journal of Physics & Chemistry of Solids, 2006, 67(9-10):2077-2082. |
| [20] Tsuneoka T , Kojima K , Bojja S . Upconversion fluorescence and low temperature fluorescence properties in Nd3+-doped ZnCl2-based glass[J]. Journal of Non-Crystalline Solids, 1996, 202(3):297-302. |
| [21] Chen X , Collins J , Dibartolo B , et al. Up-conversion luminescence of Er3+-doped yttrium, scandium, gallium garnet at different temperatures[J]. Journal of Luminescence, 1997, s 72–74(none):168-170. |
| [22] Yanhong L I , Zhang Y , Hong G , et al. Upconversion luminescence of Y_2O_3:Er~(3+), Yb~(3+) nanoparticles prepared by a homogeneous precipitation method. 稀土学报(英文版), 2008. |
| [23] Yang K , Yan L , Yu C , et al. Upconversion Luminescence Properties of Ho3+, Tm3+, Yb3+ Co-Doped Nanocrystal NaYF4 Synthesized by Hydrothermal Method[J]. Journal of Rare Earths, 2006, 24(6):757-760. |
| [24] Ganem J , Wang Y P , Boye D , et al. Nonexponential photon-echo decays of paramagnetic ions in the superhyperfine limit[J]. Physical Review Letters, 1991, 66(12):695-698. |
| [25] Errandonea D , FJ Manjón, Somayazulu M , et al. Effects of pressure on the local atomic structure of CaWO4 and YLiF4: Mechanism of the scheelite-to-wolframite and scheelite-to-fergusonite transitions[J]. Journal of Solid State Chemistry, 2004, 177(4-5):1087-1097. |
| [26] Mingyi S U , Y Zhou, Wang K , et al. Effect of Yb~(3+) concentration on upconversion luminescence of AlON:Er~(3+) phosphors. 稀土学报(英文版), 2015. |
| [27] Pei X , Hou Y , Zhao S , et al. Frequency upconversion of Tm3+ and Yb3+ codoped YLiF4 synthesized by hydrothermal method[J]. Materials Chemistry & Physics, 2005, 90(2-3):270-274. |
| [28] Lupei V , Osiac E , Sandrock T , et al. Excited state dynamics in sensitized photon avalanche processes[J]. Journal of Luminescence, 1998, s 76–77(97):441-446. |
| [29] Optically-detected nuclear quadrupole resonance of Eu 3+ LaF3 |
| [30] Research on Up-Conversion Mechanism in Er3+ /Yb3′ -Codoped Oxyfluoride Glass |




Leave a Reply